Carbyne – a straight line of carbon atoms linked by double bonds or by alternating single and triple bonds — is the next stiff, carbon-based structure with unusual and desirable properties. It has been observed under limited natural and experimental conditions, is expected to be difficult to synthesize and store, and now has been theoretically characterized.
Researchers at Rice University recently published DFT characterizations of carbyne ropes and rods, and overviews of the findings and prospects are reprinted at Phys.org:
According to the portrait drawn from calculations by Yakobson and his group:
- Carbyne’s tensile strength – the ability to withstand stretching – surpasses “that of any other known material” and is double that of graphene. (Scientists had already calculated it would take an elephant on a pencil to break through a sheet of graphene.)
- It has twice the tensile stiffness of graphene and carbon nanotubes and nearly three times that of diamond.
- Stretching carbyne as little as 10 percent alters its electronic band gap significantly.
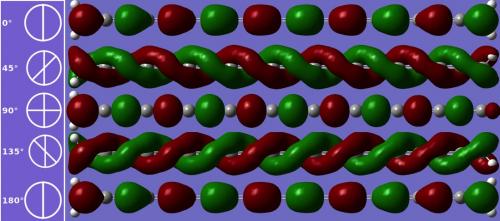
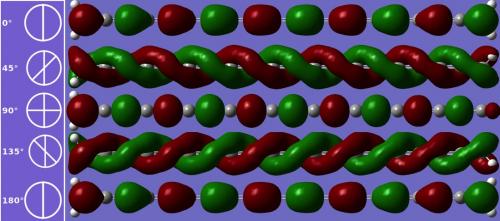
- If outfitted with molecular handles at the ends, it can also be twisted to alter its band gap. With a 90-degree end-to-end rotation, it becomes a magnetic semiconductor.
- Carbyne chains can take on side molecules that may make the chains suitable for energy storage.
- The material is stable at room temperature, largely resisting crosslinks with nearby chains.
…
Not unlike the problems of waviness in bundles of carbon nanotube, trying to utilize bundles of carbyne ropes/rods may present challenges:
The literature seemed to indicate carbyne “was not stable and would form graphite or soot,” he said.
Instead, the researchers found carbon atoms on separate strings might overcome the barrier in one spot, but the rods’ stiffness would prevent them from coming together in a second location, at least at room temperature. “They would look like butterfly wings,” Artyukhov said.
“Bundles might stick to each other, but they wouldn’t collapse completely,” Yakobson added. “That could make for a highly porous, random net that may be good for adsorption.” Artyukhov said the nominal specific area of carbyne is about five times that of graphene.
…
They’re taking a more rigorous look at the conductivity of carbyne and are thinking about other elements as well. “We’ve talked about going through different elements in the periodic table to see if some of them can form one-dimensional chains,” Yakobson said.
-Posted by Stephanie C