A select set of videos from the 2013 Foresight Technical Conference: Illuminating Atomic Precision, held January 11-13, 2013 in Palo Alto, have been made available on vimeo. Videos have been posted of those presentations for which the speakers have consented. Other presentations contained confidential information and will not be posted.
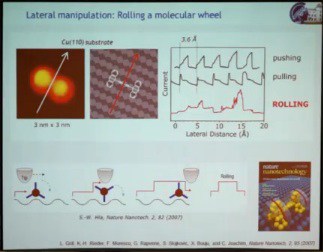
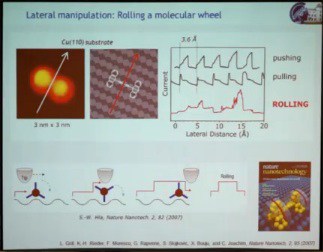
The second speaker at the Atomic Scale Devices session, the winner of the 2011 Feynman Prize for Experimental work, Leonhard Grill, presented his prize-winning work “Assembly and Manipulation of Molecules at the Atomic Scale: ‘Stiching and Switching’” biography and abstract, http://vimeo.com/62028034 – video length 27:43. He addressed two topics: (1) the manipulation of single molecules on surfaces to gain fundamental understanding of their function by triggering their function, which could be mechanical, optical, or electronic, directly on the surface; (2) how to build molecular architectures on a surface in a very controlled way with pre-defined topography using different kinds of interactions on the surface. The work he presented was done using a scanning tunneling microscope (STM) under high vacuum to keep things clean, and at the very low temperature of 5 K to minimize molecular movement. The first example illustrated a mechanical function, a rolling motion of a molecule on a surface. Previous demonstrations of lateral movement of a single molecule as a result of STM manipulation relied on getting the molecule to hop from one point on the surface to another. A molecular wheel made from a triptycene dimer was manipulated with the STM, causing the molecule to move across the surface. However, monitoring electric current through the tip in real time as the molecule moved, yielding a saw-tooth pattern, showed the motion to be hopping rather than rolling. So they changed the substrate from copper (100), which is very flat, to copper (110), which consists of atomic lines of copper atoms. Because of these corrugations on the surface, the molecular wheel was able to roll over the surface.
Azobenzene molecules were used to study optical and electronic switch functions. Azobenzene can be interconverted by light between the trans and the cis isomers, which have different shapes. Adding four bulky groups to the molecule to facilitate imaging the two isomers, single molecules of tetra-tert-butyl azobenzene were switched by electric pulses between the two isomers.
How can molecules be linked covalently on a surface? The second part of the talk focused on how to make self-organizing arrays of different types of molecules on a surface more stable, specifically, instead of supramolecular arrays held together by weak interactions, can arrays of molecules be build using covalent bonds to connect the molecules. A second advantage is that covalent bonds might allow charge transfer between the molecules. The molecular building blocks used (porphyrins) each have four legs tipped by a halogen atom (bromine) such that the weakest bond in the molecule is the bond to the halogen atom. The molecules can then be activated by heating, breaking off the halogen atoms and forming active sites through which the molecules can connect with each other on the surface, forming a very stable grid. Using similar molecules with only two legs, in the trans configuration, gives a chain instead of a grid.
However, the above is a simple one-step process. Is it possible to do a hierarchical process of sequentially linking molecules together to build more complex structures? The solution Grill presented is to use similar porphyrin building blocks with two legs trans to each other tipped with bromines and two tipped with iodine atoms. Since the iodines are more weakly bound than the bromines, the linking can be done sequentially by choosing the temperature, first at lower temperature through iodines to form chains, and then by raising the temperature through bromines to form grids. These grids are of higher quality than the ones formed using tetra-bromine building blocks, but the next step is to ask if there are unique structures that can only be formed using a two-step process. Mixing two different molecules, one square and one linear, in a two step process, you first forms chains of one type, and then chains of the second type, giving a more complex product than if they were all activated together. In another example, di-halogenated anthracene molecules were linked together on a surface and then planarized to form atomically precise graphene ribbons.
—James Lewis, PhD