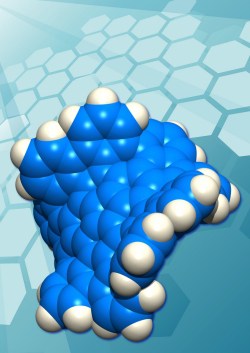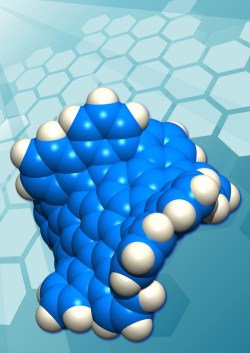
There has been a great deal of interest over the past few years in the properties of graphene, one-atom-thick sheets of trigonal carbon atoms. For the most part, the graphene sheets that have been studied are large on the molecular scale, irregular in their extent, and flat. Chemists have now synthesized distinct molecular species of grossly distorted graphene, somewhat more than one nanometer across, comprising 80 carbon atoms and 30 hydrogen atoms. A hat tip to KurzweilAI for highlighting this Boston College news release “Chemists at Boston College, Nagoya University Synthesize First Example of New Carbon Form“:
Chemists at Boston College and Nagoya University have together synthesized the first example of a new form of carbon, the team reports in the most recent edition of the journal Nature Chemistry [abstract]. This new material consists of many identical piece of grossly warped graphene, each containing exactly 80 carbon atoms joined together in a network of 26 rings, with 30 hydrogen atoms decorating the rim. These individual molecules, because they measure somewhat more than a nanometer across, are referred to generically as “nanocarbons,” or more specifically in this case as “grossly warped nanographenes.” …
Graphene sheets prefer planar, 2-dimensional geometries as a consequence of the hexagonal, chicken wire-like, arrangements of trigonal carbon atoms comprising their two-dimensional networks. The new form of carbon just reported in Nature Chemistry, however, is wildly distorted from planarity as a consequence of the presence of five 7-membered rings and one 5-membered ring embedded in the hexagonal lattice of carbon atoms.
Odd-membered-ring defects such as these not only distort the sheets of atoms away from planarity, they also alter the physical, optical, and electronic properties of the material, according to one of the principal authors, Lawrence T. Scott, the Jim and Louise Vanderslice and Family Professor of Chemistry at Boston College.
“Our new grossly warped nanographene is dramatically more soluble than a planar nanographene of comparable size,” says Scott, “and the two differ significantly in color, as well. Electrochemical measurements revealed that the planar and the warped nanographenes are equally easily oxidized, but the warped nanographene is more difficult to reduce.”
Graphene has been highly touted as a revolutionary material for nanoscale electronics. By introducing multiple odd-membered ring defects into the graphene lattice, Scott and his collaborators have experimentally demonstrated that the electronic properties of graphene can be modified in a predictable manner through precisely controlled chemical synthesis. …
From the perspectives of both potential near-term applications and potential involvement in a road from current nanotechnology to atomically precise manufacturing, these new atomically-precise structures are interesting in at least three ways. First, they illustrate the every-expanding capabilities of synthetic organic chemists. Second, the distortions from planarity caused by the 7-member and 5-member rings open possibilities for new properties arising via altered geometry. Third, these molecules are small enough that they could easily fit as molecular building blocks arrayed on some suitable scaffolding to be parts of molecular machine systems, perhaps even productive nanosystems.
—James Lewis, PhD