Protein Fragmentation Due
to Slow Highly Charged Ion Impact
by
Christiane Ruehlicke a, �,
Dieter Schneider a,
Markus Schneider a,
Robert D. DuBois b,
Rod Balhorn c
a Lawrence
Livermore National Laboratory,
Dept. of Physics and Space Technology,
Mail Stop: L-421, P.O. Box 808, Livermore, CA 94550
e-mail: [email protected],
[email protected]b
Department of Physics, University of Missouri-Rolla,
Rolla, MO 65401
e-mail: [email protected]
c Lawrence
Livermore National Laboratory,
Dept. of Biology and Biotechnology,
Mail-Stop: L-452, P.O. Box 808, Livermore, CA 94550
e-mail: [email protected]
� Christiane
Ruehlicke is affiliated with
Universit�t Bielefeld (Bielefeld, Germany) through
Rainer Hippler.
|
This is a draft paper
for a talk at the
Fifth
Foresight Conference on Molecular Nanotechnology.
The final version has been submitted
for publication in the special Conference issue of Nanotechnology.
This page uses the HTML <sup> and <sub>
conventions for superscripts and subscripts. If "103"
looks the same as "103" then your browser does not
support superscripts. If "xi" looks the
same as "xi" then your browser does not support
subscripts. Failure to support superscripts or subscripts can
lead to confusion in the following text, particularly in
interpreting exponents.
Abstract
We present first results of experiments on the fragmentation
of biomolecules using highly charged heavy ions. Fragmentation
and modification of oligopeptides, such as dimerization and
attachment of ionic salt components, have been observed by means
of mass spectrometry. Plasmid DNA molecules were imaged with an
AFM after ion irradiation and profound molecular damage was
found.
Introduction
Biomolecules such as proteins and DNA not only have important
functions in our cells but they are also being engineered for a
variety of applications in biotechnology. It is therefore
desirable to find means to modify the molecular structure of
peptides and DNA in a predictable and controlled way.
The attachment of ligands, polymerization and controlled
fragmentation are effects that various groups are investigating
via heavy ion irradiation. Fragmentation is also of interest
because of its potential application to protein sequencing, an
analytic process still limited by the availability of proteases,
which are used to cut large proteins into peptide fragments for
sequencing.
Until now, the investigation of heavy ion interaction with
biomolecules has almost exclusively been done using fission
fragments, typically with ions generated by the spontaneous
fission of 252Cf. MacFarlane and Torgerson (1976)
found that irradiation of peptides with such fission fragments
leads to fragmentation at the sites of the peptide bonds, while
Bouchonnet (1992) reported fragmentation of the sidechains in
individual amino acids.
These heavy ions carry kinetic energies that are ca. three
orders of magnitude higher than their potential or ionization
energies. Therefore most of the interaction between target and
projectile is assumed to be collisional rather than electronic.
Highly charged ions (HCI) extracted from an electron beam ion trap (EBIT) usually have
kinetic energies of a few 100 keV and they can be decelerated to
much lower kinetic energies. This enhances the electronic
interaction over the collisional one. For high ionization states
(up to U92+ ions have been produced in EBIT) the
potential energies also exceed 100 keV, which can lead to new and
exotic effects. For example experiments on highly charged ion
interactions with solid surfaces have shown new effects, such as
the emission of large clusters (up to mass m = 1000 amu for SiO2,
e.g.) and enhanced sputtering yields (Schneider and Briere,
1996). The emission of large numbers of electrons on a fs
timescale due to single ion impact has been shown to yield up to
a few 100s of electrons per incoming ion (McDonald, 1992). This
may open up the possibility to study localized, hot electron
plasma induced processes, that occur on a fs timescale. The
formation of blisterlike surface defects due to single ion impact
has been observed on mica using an atomic force microscope (AFM)
(Ruehlicke, 1995). In this context potential applications of
highly charged ions gain increasing interest also as techniques
for ion beam focusing and steering are being improved.
2. Experimental
2.1. Target Preparation
Targets used in the time of flight measurements were the
peptides RRRVAC and RVA as well as two of their constituent amino
acids R and A. The amino acids were purchased from a commercial
source (Sigma Chemical Co., St. Louis, MO) while the peptides
were custom synthesized using a PS3 Peptide Synthesizer (Rainin
Instruments) and purified by reversed-phase high performance
liquid chromatography. Aliquots of the molecules ( 50 �l of a 10
mg/ml solution in water ) were deposited on flat gold disks and
allowed to dry for 15 hours in the presence of a desiccant. The
targets were then clamped onto a sample ladder and mounted in the
experimental area within 24 hours following their preparation.
2.2 Time of Flight Spectrometry of Peptides
Time of flight secondary-ion-mass-spectrometry (TOF-SIMS) was
performed in a high vacuum (10-10 Torr) chamber
equipped for surface analysis at the end of the EBIT extraction
beamline. Highly charged ions were produced in EBIT, whose
function as an ion trap/source has been explained in detail
elsewhere (Schneider, 1991, Levine, 1989). These ions were then
extracted to use as a projectile beam. Primary ions were Xe15+,
Xe44+, Xe50+, Au65+ and Th70+,
extracted at 7 kV * q, where q is the net ion charge with fluxes
of ca. 1000 ions/s.
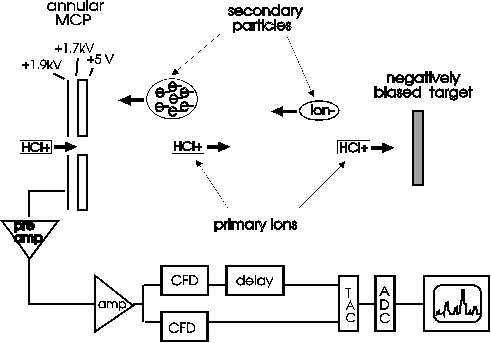
The TOF spectra were obtained using a TOF-SIMS spectrometer (Fig. 1) (Schneider and Briere, 1996). Secondary
ions are accelerated between the target and a channelplate
detector using voltages of a few kV. For negative secondary ions
the start signal was taken from electrons emitted from the sample
upon individual ion impact. For positive secondary ions protons
provided the start signal. The stop signals were given by
subsequently arriving secondary ions.
The flight time t of the secondary ions over a given distance
and in a given electric field is related to the mass/charge
ratio:
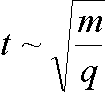
The positive secondary yield depends on the probability of
proton emission, which might follow a different projectile charge
state dependence than the larger mass secondary ions. However,
this affects the secondary ion yield only if there is no proton
emitted after primary ion impact.
Larger version (15K, 981x686 pixels)
Fig. 1: The time of flight spectrometer used here
is shown. Secondary electrons and ions are ejected from the
target and accelerated towards the microchannelplate
detector. The target is biased positive or negative depending
on which spectra are taken.
2.3. DNA Irradiation
In order to obtain images of HCI induced damage of
biomolecules, atomic force microscopy was performed on DNA
molecules. 50 �l amounts of an aqueous solution of plasmid pUC18
DNA molecules (molecular mass ca. 50000 amu, Sigma Chemicals Co.,
St. Louis, MO) were deposited on muscovite mica surfaces and
allowed to dry. The mica surfaces had been coated with
3-aminopropyltriethoxysilane (Bezanilla, 1995) to facilitate
adhesion of the DNA on the surface.
After the unirradiated samples were imaged with an AFM they
were then mounted on a sample ladder and placed in the ion
extraction beamline at a vacuum of ca. 10-8 Torr. Xe44+
ions were extracted from EBIT at 7 kV * q and directed onto the
targets. Targets were irradiated for a few hours until a total
fluence of 100 ions/�m2 was achieved.
Upon removal from the vacuum system the samples were reimaged
with the AFM. Measurements were made using a Park Scientific
Instruments LS system in contact mode.
3. Results and Discussion
3.1. Time of Flight Spectra of Peptides
Results from the TOF measurements are shown in Figures 2 - 4.
The large numbers of very low mass ions, e.g., H, C, and O
compounds, do not originate solely from the molecular sample but
also from contaminant species and the substrate and are common in
these spectra regardless of the target material. These compounds
and atomic ions of alkali metals and halides used during sample
preparation dominate the spectra up to masses of ca. 100. Mass
fragments that are unique to the peptide samples are observed in
the higher mass range.
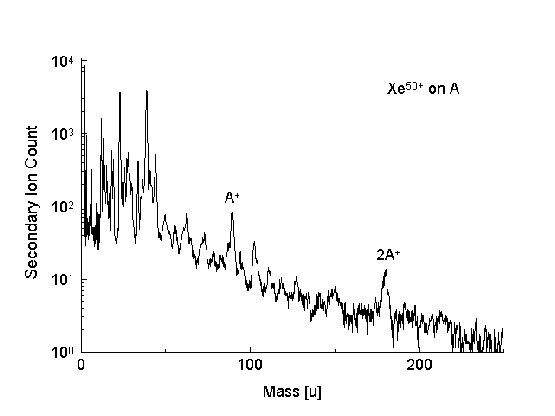
The positive secondary ion spectrum of the amino acid alanine
(Fig. 2) shows a series of mass fragments
that are both smaller and larger than the intact molecule. Most
prominent are the mass contributions of the intact molecule as
well as the molecule of twice the intact molecule mass. It is to
be noted that this mass does not correspond to the dipeptide mass
A-A, which would be 18 mass units below the double mass, since a
water molecule must be released during the formation of the
peptide bond.
Larger version (16K, 1109x832 pixels)
Fig. 2: TOF-SIMS spectrum showing the positive
secondary ion yield for the projectile ion Xe65+
on the amino acid Alanine deposited on Au.
In the case of the peptide RVA the positive spectrum (Fig. 3b) shows also a series of fragments, as
well as a significant contribution at the intact molecule mass.
The occurrence of even higher masses suggests that the intact
molecule combines with other fragments and possibly with Na,
which is present in small concentrations in the peptide solution.
The RVA spectra have been taken for two different charges of the
same primary ion: Xe15+ has a potential energy of ca.
2 keV while Xe50+ has 102 keV of potential energy.
Thus the kinetic energy differs by a factor of ca. 3, given the
dependence on the ion charge. For the same number of projectile
ions hitting the target we have found that the sputter yield is
at least one order of magnitude higher for the higher incident
charge state. The Xe15+ spectrum shows virtually no
masses larger than m = 100 amu, which indicates that higher
masses are not ejected by impact of the lower charge ion or that
their intensity is below the threshold limit. It should be noted
that the analyzing electronics used in these experiments are not
capable of recording multihits, therefore higher masses are
blocked out preferentially.
In the negative RVA spectra (Fig. 3a) the
same dependence of the secondary yields on the incident ion
charge has been observed. The spectrum taken with the higher
incident charge also shows a pronounced series of fragments with
high intensities. These fragment series seem to be generally more
pronounced in the negative spectra. A contribution at the intact
molecule mass is observed as well.
Fig. 3: Negative and positive TOF-SIMS spectra of
the tripeptide RVA deposited on gold. The incident ion was Xe50+
in this case.
We also studied the larger peptide RRRVAC, which released
molecular fragments and compounds up to mass 1000 in the negative
case (Fig. 4a). This is well beyond the mass
of the intact molecule ( m = 763 u). The positive spectrum (Fig. 4b) also shows a fragment series, but it
is much less distinct and does not extend into as high mass
regions as the negative one.
Fig. 4: Negative and positive TOF-SIMS spectra of
the peptide RRRVAC on Au, irradiated with Xe44+
ions.
3.2. Results of DNA Irradiation
AFM images reveal a profound structural change in the plasmid
DNA due to HCI irradiation (Fig. 5). HCI
impact has caused extensive fragmentation of the DNA molecules,
to the point where the circular structure of the molecules is no
longer recognizable. Some large molecular clusters have also
formed which may result from breaking the bonds that keep the DNA
attached to the mica surface, allowing multiple DNA molecules to
aggregate or interact.
Fig. 5: AFM images of plasmid DNA on mica. The
image taken before the irradiation (a) shows intact circular
plasmids, while the image taken after the irradiation (b)
shows profound molecular damage as a result of the
irradiation with Xe44+ ions.
3.3. Discussion
The underlying mechanisms for breakup and ablation are not yet
understood but it is assumed that a variety of processes occur,
which originate in the large electric field induced by the high
ion charge. This high field is likely to cause the removal of
binding electrons, therefore causing the formation and ejection
of molecular fragments. The electron depletion of the solid
substrate by the HCI is also expected to be a factor in the
ablation and ejection process, where weakening of the binding
between the molecule and the surface precedes the desorption.
Upon removal of binding electrons the molecule can change its
structure and rearrange itself to form chemical species which
would be otherwise unstable.
While the structure, binding and adhesion of the peptide
molecules differs significantly from the solid surfaces, some of
the responses to HCI impact are similar, e.g. the higher
secondary ion yields and occurrence of high mass clusters due to
HCI impact have been observed in sputtering solid surfaces with
HCI as well (Schneider and Briere, 1996). The ring shaped
circular pattern of the small fragments around clustered heavier
fragments following the breakup of the DNA may indicate a Coulomb
explosion type reaction.
4. Summary
The first results of fragmentation studies of biological
molecules by HCI are presented. TOF-SIMS spectra of peptides and
amino acids show the ablation of intact molecules as well as
fragmentation of the molecules into a number of fragments. In the
negative spectra a very pronounced series of molecular fragments
has been found to occur, while the positive spectra exhibit more
of a continuum of fragment peaks with lower relative intensities
and which occur at lower masses than in the negative case. The
ejection of molecules of twice the intact molecules' mass has
been observed and the secondary sputter yield has been shown to
increase significantly for high incident charge states. This is
consistent with findings of HCI interaction with solid surfaces.
In addition to the TOF-SIMS measurements of peptides plasmid DNA
molecules were irradiated with HCI and the resulting dramatic
molecular damage was visualized using an AFM.
5. References
Bezanilla M., Manne S., Laney D.E., Lyubchenko Y.L., et al.,
Langmuir, Feb. 1995, V 11 N 2: 655 - 659
Bouchonnet S., Denhez J.P., Hoppilliard Y., Mauriac C., Anal.
Chem. 1992, 64, 743 - 754
Levine, M.A., Marrs R.E et al., Nucl. Instr. and Meth. in
Phys. Res. B43, 1989, 431 -440
Macfarlane R.D. and Torgerson, D.F., Science, Mar. 1976, V
191, 920 - 925
McDonald, J.W., Schneider D., Clark M.W. and Dewitt, D., Phys.
Rev. Lett. 68, 1992, 2297
Ruehlicke C., Briere M.A. and Schneider D., Nucl. Instr. and
Meth. in Phys. Res. B99, 1995, 528 - 531
Schneider D. M.W. Clark, Penetrante B.M., McDonald J., DeWitt
D. and Bardsley J.N., Phys. Rev. A44 N5, 1991, 3119 - 3124
Schneider D.H.G. and Briere M.A., Physica Scripta, Vol. 53,
1996, 228 - 242
Acknowledgment
This work has been performed under the auspices of the U.S.
Department of Energy by Lawrence Livermore National Laboratory
under Contract No. W-7405-ENG-48.
� Christiane Ruehlicke is affiliated with
Universit�t Bielefeld (Bielefeld, Germany) through Rainer
Hippler.
|