Molecular Scale Electronics.
Syntheses and Testing
by
William A. Reinerth†,
LeRoy Jones II†, Timothy
P. Burgin†, Chong-wu
Zhou‡,
C. J. Muller‡, M. R.
Deshpande‡, Mark A.
Reed‡, and James M. Tour†
†Department of Chemistry and
Biochemistry, University of South Carolina, Columbia, SC,
29208, USA
‡Department
of Electrical Engineering, Yale University,
P.O. Box 208284, New Haven, CT 06520, USA
* Corresponding Author: [email protected] (James
M. Tour)
|
This is a draft paper
for a talk at the
Fifth
Foresight Conference on Molecular Nanotechnology.
The final version has been submitted
for publication in the special Conference issue of Nanotechnology.
This page uses the HTML <sup> and
<sub> conventions for superscripts and subscripts. If
"103" looks the same as "103"
then your browser does not support superscripts. If "xi"
looks the same as "xi" then your browser does not
support subscripts. Failure to support superscripts or
subscripts can lead to confusion in the following text,
particularly in interpreting exponents.
Abstract
This paper describes four significant breakthroughs in the
syntheses and testing of molecular scale electronic devices. The
16-mer of oligo(2-dodecylphenylene ethynylene) was prepared on
Merrifield's resin using the iterative divergent/convergent
approach which significantly streamlines the preparation of this
molecular scale wire. The formation of self-assembled monolayers
(SAMs) and multilayers on gold surfaces of rigid rod conjugated
oligomers that have thiol,  , , -dithiol, thioacetyl, or -dithiol, thioacetyl, or  , , -dithioacetyl end groups have been
studied. The direct observation of charge transport through
molecules of benzene-1,4-dithiol, which have been self-assembled
onto two facing gold electrodes, has been achieved. Finally, we
are reporting initial studies into what effect varying the
molecular alligator clip has on the molecule scale wire's
conductivity. -dithioacetyl end groups have been
studied. The direct observation of charge transport through
molecules of benzene-1,4-dithiol, which have been self-assembled
onto two facing gold electrodes, has been achieved. Finally, we
are reporting initial studies into what effect varying the
molecular alligator clip has on the molecule scale wire's
conductivity.
Introduction
Future computational system will likely consist of logic
devices that are ultra dense, ultra fast, and molecular-sized.1-3 The slow
step in existing computational architectures is not usually the
switching time, but the time it takes for an electron to travel
between devices. By using molecular scale electronic
interconnects,4
the transmit times could be minimized, resulting in computational
systems that operate at far greater speeds than is presently
attainable from conventional patterned architectural arrays.1 There is
another technical advantage that might also be gained from
molecular scale devices. A powerful computational system present
utilizes ca. 1010 silicon-based devices. If devices
were to be based upon single molecules,3r using routine chemical
syntheses, one could prepare ca. 1023 devices in a
single reaction flask. Of course, the task of addressing large
arrays of ordered molecular scale devices is presently
unattainable; however, the potential is certainly enough to
maintain current and future interests.
Though it is well-documented that bulk conjugated organic
materials can be semiconducting or even conducting when doped,5 we only
recently determined how thiol-ended rigid rod conjugated
molecules orient themselves on gold surfaces,6 and how we could record
electronic conduction through single undoped conjugated molecules
that are end-bound onto a metal probe surface.7 We have previously describe the
syntheses of soluble oligo(3-ethyl-2,5-thiophene-ethynylene)s and
oligo(2-alkyl-1,4-phenylene-ethynylene)s, potential molecular
scale wires, by a rapid, solution phase iterative
divergent/convergent doubling approach,8,9 as well as
the syntheses and attachments of protected thiol moieties to one
or both ends of the molecular scale wires. These thiols serve as
molecular scale alligator clips for adhesion of the molecular
scale wires to the gold probes.6,7
Herein we report four significant breakthroughs that could
have dramatic implications in the syntheses and testing of
molecular scale electronic devices. First, we have completed the
synthesis of the dodecyl-containing 16-mer on Merrifield's resin
(chloromethyl polystyrene) using the iterative
divergent/convergent approach; a method which significantly
streamlines the preparation.10
Secondly, we have studied the formation of self-assembled
monolayers (SAMs) and multilayers on gold surfaces of rigid rod
conjugated oligomers that have thiol,  , , -dithiol, thioacetyl, or -dithiol, thioacetyl, or  , , -dithioacetyl end groups. Thirdly,
molecules of benzene-1,4-dithiol have been self-assembled onto
the two facing gold electrodes of a mechanically controllable
break junction (MCB) to form a statically stable
gold-sulfur-aryl-sulfur-gold system, which allows for direct
observation of charge transport through the molecules. Finally,
we are examining what effect varying the molecular alligator clip
has on the molecule scale wire's conductivity. -dithioacetyl end groups. Thirdly,
molecules of benzene-1,4-dithiol have been self-assembled onto
the two facing gold electrodes of a mechanically controllable
break junction (MCB) to form a statically stable
gold-sulfur-aryl-sulfur-gold system, which allows for direct
observation of charge transport through the molecules. Finally,
we are examining what effect varying the molecular alligator clip
has on the molecule scale wire's conductivity.
Experiment
The monomers needed for the polymer support synthesis were
prepared as depicted in Scheme I. The
silylated triazene was divided into two portions; the first
portion was desilylated to form 1, the anchor unit to be
attached to the polymer support, while the second portion was
iodinated10
to form the iodoarene 2. Attachment of 1 to the
polymer support resin, and the oligomer syntheses on the polymer
support are depicted in Scheme II. This
polymer support approach greatly simplifies the isolation and
purification of oligomers 5, 8, 11, and 14.
Scheme I
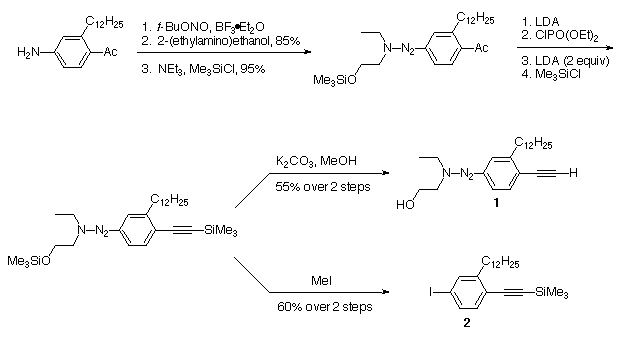
Scheme I. Synthesis of the dodecyl-containing anchor 1
and monomer 2.
Scheme II
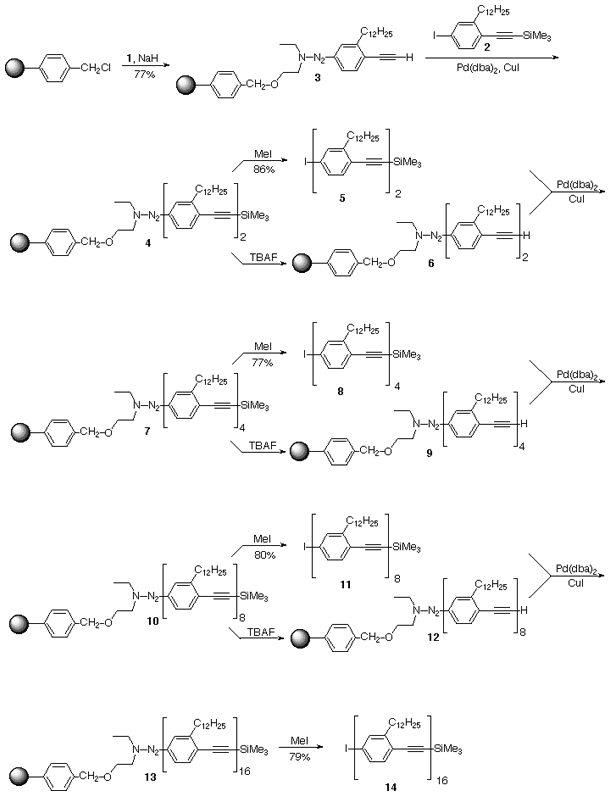
Full-sized version, 708 x 927 pixels, 13K
Scheme II. The polymer supported iterative
divergent/convergent approach to the dodecyl-containing linear
oligomers.
Results
As a prelude to the utilization of molecular scale wires in
molecular scale electronic devices, it is necessary to understand
the molecular ordering on metal surfaces. These sulfur-terminated
conjugated oligomers form self-assembled monolayers (SAMs) on
gold surfaces by attachment of the thiol end groups which serve
as molecular scale alligator clips.6a
The SAMs were analyzed using ellipsometry, X-ray photoelectron
spectroscopy (XPS), and infrared external reflectance
spectroscopy. The thiol moieties usually dominate adsorption on
the gold sites; interactions with the conjugated  -systems are weaker. Rigid rod -systems are weaker. Rigid rod  , , -dithiols form assemblies in which one
thiol group binds to the surface while the second thiol moiety
projects upward at the exposed surface of the SAM. In situ
deprotection of the thiol moieties by deacylation of thioacetyl
groups using NH4OH permits formation of SAMs without
having to isolate the oxidatively unstable free thiols. Moreover,
direct adsorption, without exogenous base, of the
thioacetyl-terminated oligomers can be accomplished to generate
gold surface-bound thiolates. However, in the non-base-promoted
adsorptions, higher concentrations of the thioacetyl groups,
relative to that of thiol groups, are required to achieve
monolayer coverage in a given interval. A thiol-terminated
phenylene ethynylene system was shown to have a tilt angle of the
long molecular axis of <20° from the normal to the substrate
surface. These aromatic -dithiols form assemblies in which one
thiol group binds to the surface while the second thiol moiety
projects upward at the exposed surface of the SAM. In situ
deprotection of the thiol moieties by deacylation of thioacetyl
groups using NH4OH permits formation of SAMs without
having to isolate the oxidatively unstable free thiols. Moreover,
direct adsorption, without exogenous base, of the
thioacetyl-terminated oligomers can be accomplished to generate
gold surface-bound thiolates. However, in the non-base-promoted
adsorptions, higher concentrations of the thioacetyl groups,
relative to that of thiol groups, are required to achieve
monolayer coverage in a given interval. A thiol-terminated
phenylene ethynylene system was shown to have a tilt angle of the
long molecular axis of <20° from the normal to the substrate
surface. These aromatic  , , -dithiol-derived monolayers provide the basis for
studies leading to the design of molecular wires capable of
bridging proximate gold surfaces. -dithiol-derived monolayers provide the basis for
studies leading to the design of molecular wires capable of
bridging proximate gold surfaces.
Charge transport in and the measurement of the conductance of
single organic molecules is an intriguing, experimentally
challenging, and long sought goal. These measurements have been
performed on benzene-1,4-dithiolate connected between stable
proximal metallic gold contacts using a mechanically controllable
break junction (MCB).11
The metal-molecule-metal configuration presents the molecular
embodiment of a system analogous to a quantum dot, with the
potential barriers replaced by the contact barrier of the
gold-thiolate endgroups (Figure 1). The
results show a highly reproducible apparent Coulomb gap at about
0.7 V at room temperature. This study provides a direct,
quantitative measurement of the molecular conductance of a
junction containing a single molecule, a fundamental step in the
emerging field of molecular scale electronics.
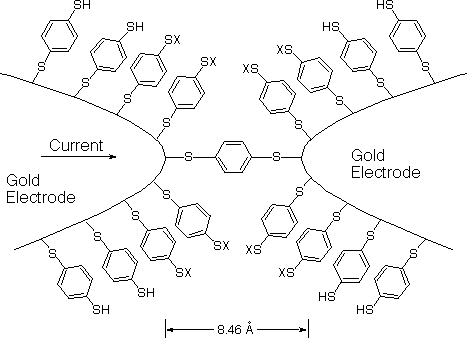
Figure 1. The desired placement of
benzene-1,4-dithiolate between gold electrodes in a MCB. (X =
H, Au)
By measuring the conductivity of a series of molecules that
are systematically altered, the contribution of each component of
a molecular scale electronic device can be determined. For
example, the simplest experiment of this type would be to measure
the conductivity of identical arrays of molecular wires in which
only the wires' attachment to the gold surface is altered. Thus,
by varying the molecular alligator clip and examining the
molecules' conductivity, the contribution of the alligator clip
to the resistance of the molecular wire can be determined. Our
initial work has focused on varying the alligator clip by
proceeding down the chalcogens from S to Se to Te.
We first desired a stable, yet easily removable, protecting
group for selenium that would allow us to use the same in situ
deprotection prototcol we had employed for the thioacetates. An
investigation of several protecting groups led us to conclude
that, as with sulfur, the acetyl group was the protecting group
of choice.12
Recently, molecular arrays of both 4,4'-biphenyl thioacetate and
4,4'-biphenyl selenoacetate have been subjected to conductivity
examination using an
evaporated-metal-top-contact/molecules/metallic-bottom-contact
configuration (X = S, Se in Figure 2). It
appears that the selenium alligator clip creates a higher barrier
for electron transport regardless of the direction of electron
flow.13
This is puzzling in light of the greater metallic character of Se
versus S which we reasonably assumed would yield a lower barrier
for Se. This is also in disagreement with recent theoretical
calculations which indicate that the barrier height for selenium
should be significantly lower than for sulfur.14 Additional work is underway to
explain this unexpected result and examine the corresponding
tellurium compound.
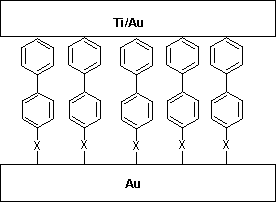
Figure 2. System for measuring conductivity of a
molecular array. (X = molecular alligator clip)
Conclusions
In summary, we have made four significant breakthroughs in the
syntheses and testing of molecular scale electronic devices.
First, we have completed the synthesis of the dodecyl-containing
16-mer on Merrifield's resin (chloromethyl polystyrene) using the
iterative divergent/convergent approach; a method which
significantly streamlines the preparation. Secondly, we have
studied the formation of self-assembled monolayers (SAMs) and
multilayers on gold surfaces of rigid rod conjugated oligomers
that have thiol,  , , -dithiol, thioacetyl, or -dithiol, thioacetyl, or  , , -dithioacetyl end groups. Thirdly,
molecules of benzene-1,4-dithiol have been self-assembled onto
the two facing gold electrodes of a mechanically controllable
break junction (MCB) to form a statically stable
gold-sulfur-aryl-sulfur-gold system, which allows for direct
observation of charge transport through the molecules. Finally,
we are investigating what effect varying the molecular alligator
clip has on the molecule scale wire's conductivity.15 -dithioacetyl end groups. Thirdly,
molecules of benzene-1,4-dithiol have been self-assembled onto
the two facing gold electrodes of a mechanically controllable
break junction (MCB) to form a statically stable
gold-sulfur-aryl-sulfur-gold system, which allows for direct
observation of charge transport through the molecules. Finally,
we are investigating what effect varying the molecular alligator
clip has on the molecule scale wire's conductivity.15
References
1. For some recent background work on
molecular scale electronics, see: (a) Molecular
Electronics: Science and Technology, Aviram, A., Ed.;
Confer. Proc. No. 262, American Institute of Physics: New York,
1992. (b) Miller, J. S. Adv. Mater. 1990, 2,
378, 495, 601. (c) Molecular and Biomolecular Electronics,
Birge, R. R., Ed.; Advances in Chemistry Series 240, American
Chemical Society, 1991. (d) Nanostructures and Mesoscopic
Systems, Kirk, W. P.; Reed, M. A., Eds.; Academic: New
York, 1992.
2. For some theoretical considerations on molecular scale
wires, see: (a) Samanta, M. P.; Tian, W.; Datta, S.; Henderson,
J. I.; Kubiak, C. P. Phys. Rev. B 1996, 53,
R7626. (b) Mujica, V.; Kemp, M.; Roitberg, A.; Ratner. M. J.
Phys. Chem. 1996, 104, 7296. (c) Joachim,
C.; Vinuesa, J. F. Europhys. Lett. 1996, 33,
635.
3. For some recent background work on the formation of
molecular-based transporters and devices, see: (a) Grosshenny,
V.; Harriman, A.; Ziessel R. Angew. Chem. Int. Ed. Engl.
1995, 34, 1100. (b) Langler, L.; Stockman, L.;
Heremans, J. P.; Bayor, V.; Olk, C. H.; Van Haesendonck, C.;
Bruynseraede, Y.; Issi, J. -P. Synth. Met. 1995,
70, 1393. (c) Pascual, J. I.; Méndez, J.; Gómez-Herrero,
J.; Baró, A. M.; Garcia, N.; Landman, U.; Luedtker, W. D.;
Bogachek, E. N.; Cheng, H.-P. Science 1995, 267,
1793. (d) Purcell, S. T.; Garcia, N.; Binh, V. T.; Jones, L., II;
Tour, J. M. J. Am. Chem. Soc. 1994, 116,
11985. (e) Martin, C. R. Science 1994, 266,
1961. (f) Seth, J.; Palaniappan, V.; Johnson, T. E.; Prathapan,
S.; Lindsey, J. S.; Bocian, D. F. J. Am. Chem. Soc. 1994,
116, 10578. (g) Gust, D. Nature 1994, 372,
133. (h) Wu, C.; Bein, T. Science 1994, 266,
1013. (i) Wagner, R. W.; Lindsey, J. S.; Seth, J.; Palaniappan,
V.; Bocain, D. F. J. Am. Chem. Soc. 1996, 118,
3996. (j) Sailor, M. J.; Curtis, C. L. Adv. Mater. 1994,
6, 688. (k) Brigelletti, F.; Flamigni, L.; Balzani, V.;
Collin, J.; Sauvage, J.; Sour, A.; Constable, E. C.; Thompson, A.
M. W. C. J. Am. Chem. Soc. 1994, 116,
7692. (l) Wu, C.; Bein, T. Science 1994, 264,
1757. (m) Moerner, W. E. Science 1994, 265,
46. (n) Sessler J. L.; Capuano, V. L.; Harriman, A. J. Am.
Chem. Soc. 1993, 115, 4618. (o) Farazdel,
A.; Dupuis, M.; Clementi, E.; Aviram. A. J. Am. Chem. Soc.
1990, 112, 4206. (p) Tour, J. M.; Wu, R.; Schumm,
J. S. J. Am. Chem. Soc. 1991, 113,
7064. (q) Dai, H.; Wong, E. W.; Lieber, C. M. Science
1996, 272, 523. (r) Wu, R.;
Schumm, J. S.; Pearson, D. L.; Tour, J. M. J. Org. Chem.
1996, 61, 6906. (s) Ward, M. D. Chem. Ind.
1996, 569.
4. "Molecular electronics" is a
poorly defined term since some authors refer to it as any
molecular-based system such as a film or a liquid crystalline
array. Other authors, including us, have preferred to reserve the
term "molecular electronics" for single molecule tasks,
such as single molecule-based devices or single molecular wires.
Due to this confusion, we have chosen here to follow the Petty et
al. (Introduction to Molecular Electronics,
Petty, M. C.; Bryce, M. R.; Bloor, D., Eds.; Oxford Univ. Press:
New York, 1995) terminology by using two subcategories, namely
"molecular materials for electronics" for bulk
applications and "molecular scale electronics" for
single molecule applications.
5. Handbook of Conducting Polymers,
Skotheim, T. A., Ed.; Dekker: New York, 1986.
6. (a) Tour, J. M.; Jones, L., II; Pearson,
D. L.; Lamba, J. S.; Burgin, T.; Whitesides, G. W.; Allara, D.
L.; Parikh, A. N.; Atre, S. J. Am. Chem. Soc. 1995,
117, 9529. (b) Dhirani, A.; Zehner, R. W.; Hsung, R. P.;
Guyot-Sionnest, P., Sita, L. R. J. Am. Chem. Soc. 1996,
118, 3319.
7. Bumm, L. A.; Arnold, J. J.; Cygan, M.
T.; Dunbar, T. D.; Burgin, T. P.; Jones, L., II, Allara, D. L.;
Tour, J. M.; Weiss, P. S. Science 1996, 271,
1705.
8. (a) Schumm, J. S.; Pearson, D. L.; Tour,
J. M. Angew. Chem. Int. Ed. Engl. 1994, 33,
1360. (b) Pearson, D. L.; Schumm, J. S.; Tour, J. M. Macromolecules
1994, 27, 2348. (c) Pearson, D. L.; Tour, J. M. J.
Org. Chem. 1997, 62, 1376.
9. Jones, L., II; Schumm, J. S.; Tour, J.
M. J. Org. Chem. 1997, 62, 1388.
10. (a) Young, J. K.; Nelson, J. C.;
Moore, J. S. J. Am. Chem. Soc. 1994, 116,
10841. (b) Bharathi, P.; Patel U.; Kawaguchi, T.; Pesak, D. J.;
Moore, J. S. Macromolecules 1995, 28,
5955. (c) Polymer-Supported Reactions in Organic Synthesis,
Hodge, P.; Sherrington, D. C., Eds.; Wiley: New York, 1980. (d)
Nelson, J. C.; Young, J. K.; Moore, J. S. J. Org. Chem.
1996, 61, 8160.
11. Reed, M. A.; Zhou, C.; Muller, C. J.;
Burgin, T. P.; Tour, J. M. Science in press.
12. Reinerth, W. A.; Tour, J. M. J.
Org. Chem. Manuscript in preparation.
13. Reed, M. A.; Zhou, C.; Reinerth, W.
A.; Tour, J. M. Unpublished results.
14. Tour, J. M.; Seminario, J. Unpublished
results.
15. We thank the Defense Advanced Research
Projects Agency for support of this work.
|