Modeling the piston-like movement in a rotaxane-based molecular machine
Department of Chemistry, University of Joensuu,
FIN-80101 Joensuu, Finland
This is an abstract
for a presentation given at the
11th
Foresight Conference on Molecular Nanotechnology
The possibility to manufacture synthesizable and controllable nanoscale devices has opened up an active area of research. Rotaxanes, pseudorotaxanes and other similar inclusion complexes offer a series of attractive probe molecules to mimic macroscopic devices, such as machines and switches, in a molecular scale1. The aim of our work is to investigate a piston-like movement of a organic guest in a rotaxane-based molecular machine. We have selected an inclusion complex between 1,5-dimethoxynaphthalene and cyclobis(paraquat-p-phenylene), which is one of the experimentally known host-guest systems,2,3 to simulate the process.
It has been found, that the overall process of threading and dethreading of the guest can be modulated by changing the chemical environment of the system. However, experimentally the detailed analysis of the piston movement is a complicated task. Computational methods provide effective tools for a systematic study of the sophisticated operations carried out by molecular machines.
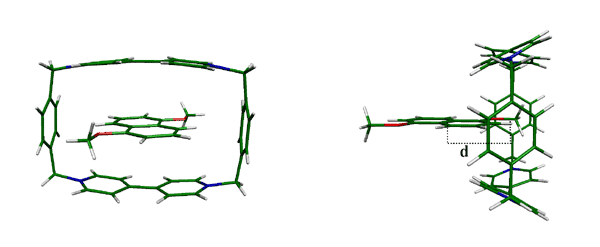
To understand the energetics behind the process, we modeled the potential energy behavior of 1,5-dimethoxynaphthalene/cyclobis(paraquat-p-phenylene) system by investigating the dethreading of the guest from the rotaxane macrocycle. The dethreading process was followed by monitoring the change of distance (d) measured from the center of the piston molecule to the center of the macrocycle ( Figure 1). The potential energy maximum was found at a distance of 2.0 Å. This corresponds to the primary energy barrier, which has to be overcome to operate the machine. The effect of the movement on the geometrical parameters of the macrocycle was also studied.
References
- Shipway, A. N. and Willner, I., Acc. Chem. Res. 34 (2001) 421.
- Odell, B., Reddington, M.V., Slawin, A. M. Z., Spencer, N., Stoddart, J. F., and Williams, D. J., Angew. Chem. 100 (1988) 1605.
- Asakawa, M., Dehaen, W., L�abbe, G., Menzer, S., Nouwen, J., Raymo, F. M., Stoddart, J. F. and Willliams, D. J., J. Org. Chem. 61 (1996) 9591.
Abstract in Microsoft Word® format 1,755,462 bytes
*Corresponding Address:
Pipsa Hirva
Department of Chemistry, University of Joensuu
P.O.Box 111, FIN-80101 Joensuu, Finland
Phone: +358-13-2513333 Fax: +358-13-2513390
Email: [email protected]
|