Laser Assisted Deposition Of Bacteriorhodhopsin Assemblies
by
Gunjan Agarwal* and Ratna S. Phadke
Chemical Physics Group, Tata Institute of Fundamental Research, Homi Bhabha Road, Mumbai 400 005, India
*Present Address for corresponding author:
Biophysics Department, Albert Einstein College of Medicine
1300 Morris Park Ave., Bronx, NY 10461
Phone: (718) 430 2109, Fax: (718) 430 8819, Email: [email protected]
This is a draft paper for the
Sixth
Foresight Conference on Molecular Nanotechnology.
The final version has been submitted
for publication in the special Conference issue of Nanotechnology.
Abstract
Supramolecular assemblies of bacteriorhodhopsin (bR) have been formed by incorporating bR in a matrix of L-a
-phosphatidylcholine distearoyl (DSPC) molecules. The preformed assemblies have been physically lifted and deposited onto solid substrates by the laser assisted deposition (LAD) technique. The bR assemblies thus deposited have been characterized using a variety of techniques like absorption studies, optical microscopy and X-ray diffraction. The structural and functional properties of the bR assemblies are found to be preserved after LAD.
Introduction
Interfacing of individual and intact preformed supramolecular assemblies of biomolecules onto a solid substrate is important as such molecular assemblies can serve as self-contained single modules for fabrication of molecular devices. The process of deposition should be such that the structural and functional properties of biomolecular assemblies are preserved after being interfaced onto solid substrates. Laser assisted deposition (LAD) has been established as a unique tool not only for formation of thin films of inorganic materials but also for investigating and processing of inorganic and organic materials [Crisey et al; Braren et al]. It has also been demonstrated that pulsed laser deposition can be successfully utilized for fabrication of nanostructures [Morales et al, 1995]. The main advantages of LAD are as follows. The method is free of solvent-solute and solute-substrate interactions, which are the controlling factors in spin-drying [Chinn et al, 1994] or the Langmuir-Blodgett technique [Langmuir, 1917]. Moreover, LAD offers a possibility of arranging preformed assemblies in a well-defined architecture by physically lifting and depositing the molecular assemblies onto solid surfaces.
Organic and biological macromolecules are known to be susceptible to thermal degradation. It is therefore desirable to entrap these molecules in a highly absorbing matrix consisting of molecules with long hydrocarbon chains such as detergents or lipids. Use of such matrix has been introduced in 1987 for matrix assisted laser desorption and ionization (MALDI) experiments on biopolymers [Karas et al 1987, 1996]. The matrix helps in a controlled transfer of laser energy to the entrapped functional and often fragile molecules such as enzymes. The matrix provides additional benefit as lipids or detergents are known to be imbued with self-assembling properties and capacity to readily encapsulate molecules [Phadke et al, 1996; Jain et al, 1980]. In addition, use of the matrix helps to prevent aggregation of the functional molecules.
In our earlier works we have demonstrated the use LAD technique to deposit preformed supramolecular assemblies of biomolecules onto a solid substrate, namely: (1) the enzyme glucose oxidase embedded in the surfactant sodium dodecyl sulphate [Phadke et al, 1998] and (2) riboflavin incorporated in phospholipids [Agarwal et al, 1998]. We report here laser assisted deposition of supramolecular assemblies of the photosensitive protein bacteriorhodhopsin (bR) embedded in the lipid L-a
-phosphatidylcholine distearoyl (DSPC). bR is a component of the purple membrane (PM) of Halobacterium halobium, and functions as a light driven proton pump [Oesterhelt et al, 1974; Packer, 1982]. It undergoes a cyclic photochemical reaction and has potential applications for photochromic [Vsevolodov et al, 1989], holographic [Hampp et al, 1990], non-linear optical [Birge, 1990] and information processing devices [Oesterhelt et al, 1991].
Materials and methods
Purple membrane (PM) suspensions containing bacteriorhodhopsin (bR) have been kindly gifted by Dr. H. Weetall, NIST, USA. The bR + PM suspension has been incorporated in the lipid L-a-phosphatidylcholine distearoyl (DSPC) (1:50 w/w) using the standard method of forming liposomes [Szoka et al, 1981]. DSPC has been purchased from Sigma chemicals company. Doubly distilled de-ionized water has been used. Aliquots of aqueous dispersion of liposomes are evenly spread onto one side of thick stainless steel discs and allowed to dry in a dessicator. The procedure is repeated several times to get sufficiently thick coating (»
0.5 mm) of the material. This coated material serves as the target for LAD. The stainless steel discs are found useful for heat dissipation. A part of the liposomal dispersion has also been coated on glass slides by spin drying to serve as control samples for comparative assessments of the LAD material. Care has been taken while handling bR samples and the test-tubes, dessicators etc. have been kept covered to avoid exposure to light.
Clean glass and quartz slides have been used as substrates. They have been specially cut to a size such that they can be easily inserted in a 1 ml cuvette for absorption studies. LAD has been carried out in a small chamber maintained at rotary vaccum of 10-3 atm. The target-substrate distance has been kept as 2 cm. The target is rotated to avoid constant exposure of the laser beam at one spot. Nanosecond pulsed Lambda Physik LPX 200 excimer laser has been used at 305 nm wavelength. Incident energy of the laser beam on the target has been varied from 5 to 12 mJ and the best results have been obtained at 10 mJ. The laser beam is de-focussed to a spot size of approximately 0.5 mm diameter, so that the resultant fluence is low. The repetition rate of the laser beam has been varied from 1 Hz to 100 Hz. At 1 Hz, the yield of deposition is too low to form good quality films. As the repetition rate is increased, the rate of material deposited on to the substrate also increases. At repetition rates of more than 10 Hz, charring of the target material takes place and at 100 Hz, the material is almost completely burnt. We found that the best deposition is obtained at the repetition rate of 5 Hz.
Absorption spectra have been recorded using a Shimadzu UV 2100 double beam spectrophotometer. Optical microscope used is Leitz Aristomet from Leica Instruments. JEOL JDX 8030 has been used for X-ray diffraction studies. The Ka (l = 1.541 ± 0.20 Å) line from Cu target is used as the incident X-ray.
Characterization of bR assemblies deposited using LAD
Absorption studies
The assay of the ablated material has been made using absorption studies [Oesterhelt et al, 1973 a,b]. bR is known to strongly absorb at 570 nm, characteristic of the retinal chromophore. Absorption spectra of bR supramolecular assemblies deposited on quartz slides have been recorded. A clean quartz slide served as the reference in the double beam absorbance spectrometer. Figure 1a shows the absorption spectra of LAD material of bR in DSPC with a maxima at 570 nm. This is characteristic of the "B" state of the bR photocycle. For assay of bR, the LAD samples are equilibrated with ether vapours for 30 minutes and the absoprtion spectrum is recorded (Figure 1b). It can be seen that the 570 nm absorption peak decreases and another peak around 490 nm develops. This is due to the formation of the 460 to 490 nm complex in bR in the presence of ether [Oesterhelt et al, 1973 a]. It is in this state that reversible photo-bleaching of bR can be accomplished on exposure to light.
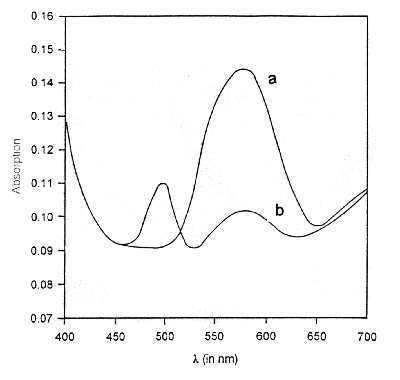
Figure 1 Absorption spectra of laser assisted deposition of bR organizates on quartz slides a before and b after subjecting to ether vapours. The 570 nm peak characteristic of the "B" state of bR is present in a. Addition of ether vapours leads to partial quenching of the 570 nm peak and appearance of a 490 nm peak as in b. In this state bR can be reversibly photo-bleached.
The samples saturated with ether vapors have been exposed to white light from a 60 W mercury bulb for 2 minutes. Upon illumination, the purple colour changes to yellow. This is characteristic of the "M" state in the bR cycle. The purple colour is restored after the sample is left in dark for a few minutes. For time-dependent studies, a time series of absorption spectra has been recorded immediately after the exposure of bR films to white light, for every 1 second time interval [Oesterhelt et al, 1973 b]. These spectra have been subtracted from the bR spectrum (in dark), to obtain a set of difference spectra (Figure 2). The difference spectra show a negative peak at 570 nm and a positive peak at 412 nm. The magnitudes of both these peaks decay with time indicating that the 412 nm component decays in time with the formation of 570 nm component. The absorbance at 570 nm and 412 nm has been plotted as a function of time (Figure 3a). The semi-logarithmic plot of these curves are parallel straight lines (Figure 3b). The half-time of the 570 and 412 nm states has been estimated from the slope of these lines as 25 s, under the given experimental conditions. It can be seen both the peaks transform synchronously, indicating that these correspond to bR undergoing its photo-cycle. The relative magnitude of the 412 nm peak is 0.5 times the 570 nm peak which is consistent with the relative quantum yield at 412 nm. The isosbetic point reported at 462 nm [Oesterhelt, D. 1973b] could not be observed due to the noise in the spectra which results from scattering of light in case of solid samples.
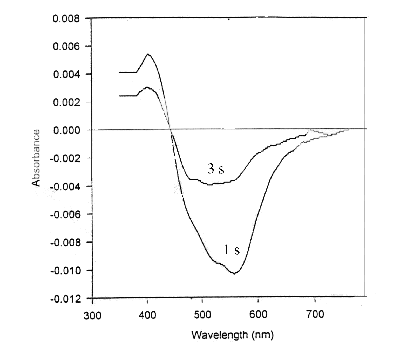
Figure 2. Difference spectra obtained by subtracting absorption spectrum of LAD of bR bleached with white light from the "dark" spectrum, after 1 and 3 seconds after exposure to light. A negative 570 nm peak and a positive 412 nm peak are obtained, characteristic of "B" and "M" states, respectively. Also the magnitude of 412 nm peak is almost half of the 570 nm peak.
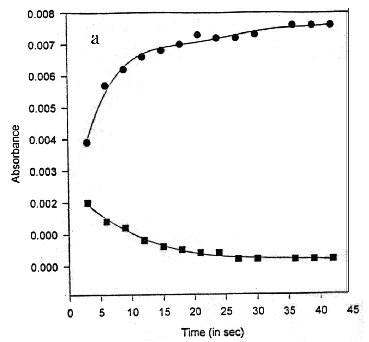
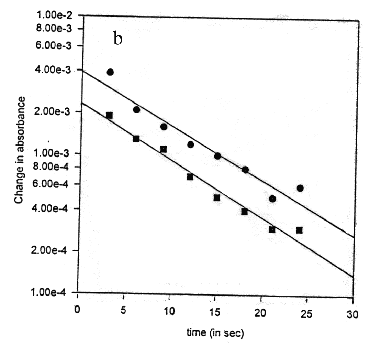
Figure 3. Kinetics of regeneration reaction after bleaching a time course of absorbance at 570 (l, circles) and 412 (n, squares) nm b half-logarithmic plot of the data in a demonstrating identical lifetimes of 25 s for both components
Optical microscopy
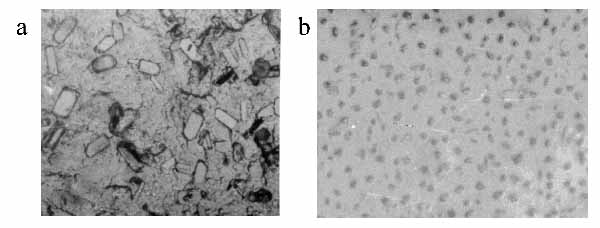
Figure 4a and b show the optical microscope photographs of supramolecular assemblies of bR prepared using spin-drying and LAD respectively. While the spin-dried sample is heterogeneous in its composition, LAD of bR supramolecular assemblies shows a homogenous distribution of uniform sized supramolecular assemblies 5-10 µm in size. The interspacings were measured for 100 bR assemblies and a graph of interspacing vs. frequency of occurrence has been plotted. The supramolecular assemblies are evenly spaced as is evident from the distribution of their spacings with the peak around 15 µm (Figure 5).
Full size Fig. 4 (495K)
Figure 4. Optical microscope photographs of preformed assemblies of bR in DSPC a spin-dried and b LAD. A heterogeneous distribution of particle shape and size is present in a. In b uniform sized assemblies (5-10 µm size) are homogeneously dispersed.
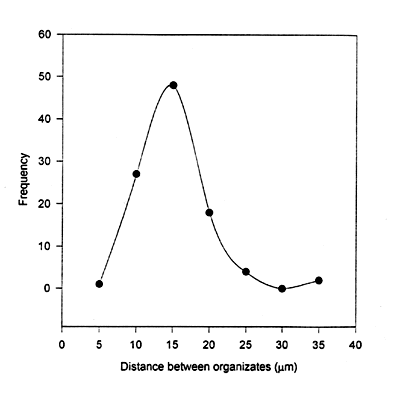
Figure 5. Spatial distribution of the bR assemblies in a LAD sample
X-ray diffraction
Low angle X-ray diffraction of bR supramolecular assemblies has been carried out on spin-dried and LAD films. XRD peaks upto seventh order are present, with some missing orders due to low intensities. The data shows a repeat distance of 32 Å (Table 1) which corresponds to length of a-helices in bR. bR is known to consist of seven transmembrane a-helices and it has been shown by XRD studies that the length of each a-helix is 32 Å
[Henderson, 1975; Blaurock, 1975].
Table 1: Low angle XRD data for films of bR assemblies
|
Peak order (n) |
2q
values (in degrees) |
| |
Spin drying |
LAD |
|
2 |
5.54 |
5.48 |
|
3 |
8.30 |
8.32 |
|
6 |
16.64 |
17.0 |
|
7 |
19.50 |
19.28 |
|
a
-helix length |
31.90 ±
0.20 Å
|
31.90 ±
0.20 Å
|
Conclusions
Supramolecular assemblies of bR could be physically lifted and deposited onto solid substrates by means of LAD. The absorbance studies indicate that bR retains its functional integrity after LAD. Morphology of the bR assemblies thus deposited is homogenous and uniform. The good quality of the films thus formed indicates that LAD can be effectively used to interface supramolecular assemblies of complex and/or functional biomolecules onto solid substrates. The films can also be made with desired molecular orientation and architecture with the help of external electric fields and patterning can be achieved by use of suitable masks.
In another set of experiments, bR in PM without the DSPC matrix was subject to LAD. In this case the protein showed no activity indicating that it had possibly got charred or denatured. Hence the use of a matrix is essential for LAD of biomolecules.
Acknowledgements
We are grateful to Dr. E. Krishnakumar, TIFR, Mumbai for providing us with his laser facility.
References:
- Agarwal, G.; and Phadke, R. S. (1998) Materials Science and Engineering C 6(1), pages13-17
- Birge, R. R. (1990) Annu. Rev. Phys. Chem. 41, pages 683-733
- Blaurock, A. E. (1975) J. Mol. Biol. 93, pages 139-158
- Braren, B.; Dubowski, J. J.; and Norton, D. P. (eds) Laser Ablation in Materials Processing: Fundamentals and Applications, Mater. Res. Soc. Symp. Proc. Vol 285
- Chinn, D.; and Janata, J. (1994) Thin Solid Films 252, pages 145-151
- Crisey D. B.; and Hubler G. K. (1994) Pulsed Laser Deposition of Thin Films, John Wiley
- Hampp, N.; Brauschle, C.; and Oesterhelt, D. (1990) Biophysical Journal 58, pages 83-93
- Henderson, R. (1975) J. Mol. Biol. 93, pages 123-138
- Jain, M. K.; and Wagner, R. C. (1980) Introduction to Biological Membranes, John Wiley, pages 38-39
- Karas, M.; Bachmann, D.; Bahr, U.; and Hillenkamp, F. (1987) Int. J. Mass. Spectrom. Ion Proc. 78, pages 53-68
- Karas, M. (1996) Biochem. Mass Spectrom. 24, page 897
- Langmuir, I. (1917) J. Am. Chem. Soc. 39, pages 1848-1906
- Morales, P.; Pavone, A.; Sperandei, M.; Leter, G.; Mosiello, L.; Nencini, L.; Grifoni, L; and Santucci, S. (1995) Mater. Sci. and Engg. C2, pages 173-179
- Oesterhelt, D.; Meentzen, M.; and Schuhmann, L. (1973a) Eur. J. Biochem. 40, pages 453-463
- Oesterhelt, D.; and Hess, B. (1973b) Eur. J. Biochem. 37, pages 316-326
- Oesterhelt, D.; and Stoeckenius, W. (1974) Methods in Enzymology 31, pages 667-678
- Oesterhelt, D.; Brauchle, C.; and Hampp, N.(1991) Quart. Rev. of Biophy. 24, No.4, page 425
- Packer, L. (1982) Methods in Enzymology 88, sections 1-27
- Phadke, R. S.; and Agarwal, G. (1996), Mol. Cryst. Liq. Cryst. 288, pages 119-128
- Phadke, R. S.; and Agarwal, G. (1998) Materials Science and Engineering C 5 (3-4), pages 237-241
- Szoka, F.; and Papahadjopoulos, D. (1981) Liposomes: From Physical Structure To Therapeutic Application (Elsevier/North-Holland Biomedical Press), pages 54-56
- Vsevolodov, N. N.; Druzhko, A. B.; and Djukova, T. V. (1989) Moleular Electronics: Biosensors and Biocomputers, Hong, F. T. (ed), Plenum Press, NY, page 381
|