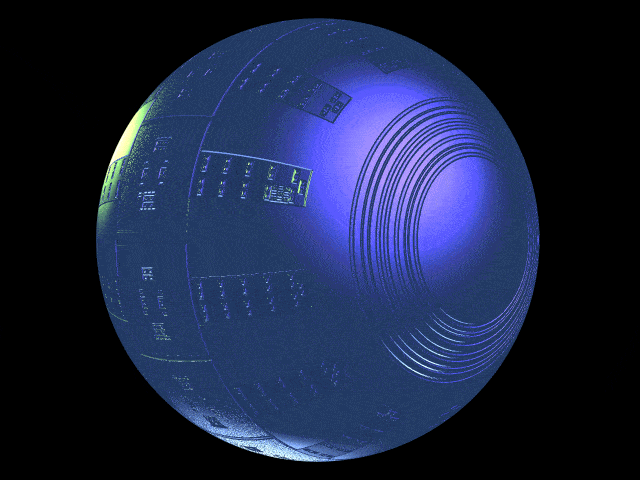

Defective Respirocyte
1999
640 X 480 pixels
Color
The image shows a defectively manufactured respirocyte with intact sensors and bar code, but missing the entire sorting rotor subsystem. Quality control in manufacturing is essential and may be strictly monitored by the FDA. Such defective devices should be very easy to detect and remove during the manufacturing process.
© Copyright 1999 Interworld Productions, LLC, P.O. Box 30121, Seattle, WA 98103. For reprint permission, please contact Forrest Bishop at [email protected]. The Respirocyte was conceived and designed by Robert A. Freitas Jr., whose permission is also required.
Robert A. Freitas Jr., “Respirocytes in Nanomedicine,” Graft: Organ and Cell Transplantation 52(May 2050):153, Fig. 5 (special “Future Issue”).
None.