Visionary proposals for advanced medical nanorobots often picture µm scale submarine-like devices navigating the bloodstream. Those are probably still a couple decades away, but prototypes of conceptually much simpler six µm scale motors that could someday navigate the oceans to sequester carbon dioxide have been demonstrated. A hat tip to Science Daily for reprinting this news release from the University of California at San Diego Jacobs School of Engineering”Tiny carbon-capturing motors may help tackle rising carbon dioxide levels“:
Machines that are much smaller than the width of a human hair could one day help clean up carbon dioxide pollution in the oceans. Nanoengineers at the University of California, San Diego have designed enzyme-functionalized micromotors that rapidly zoom around in water, remove carbon dioxide and convert it into a usable solid form.
The proof of concept study represents a promising route to mitigate the buildup of carbon dioxide, a major greenhouse gas in the environment, said researchers. The team, led by distinguished nanoengineering professor and chair Joseph Wang, published the work this month in the journal Angewandte Chemie [abstract].
“We’re excited about the possibility of using these micromotors to combat ocean acidification and global warming,” said Virendra V. Singh, a postdoctoral scientist in Wang’s research group and a co-first author of this study.
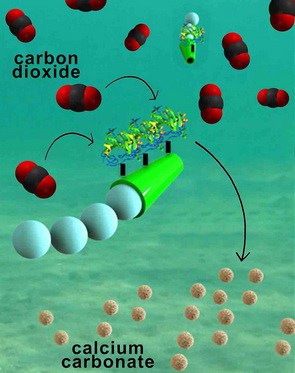
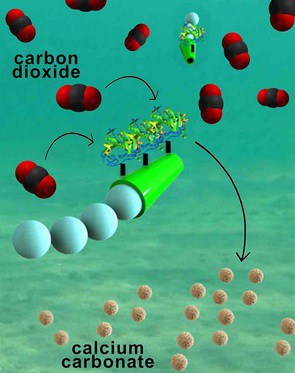
In their experiments, nanoengineers demonstrated that the micromotors rapidly decarbonated water solutions that were saturated with carbon dioxide. Within five minutes, the micromotors removed 90 percent of the carbon dioxide from a solution of deionized water. The micromotors were just as effective in a sea water solution and removed 88 percent of the carbon dioxide in the same timeframe.
“In the future, we could potentially use these micromotors as part of a water treatment system, like a water decarbonation plant,” said Kevin Kaufmann, an undergraduate researcher in Wang’s lab and a co-author of the study.
The micromotors are essentially six-micrometer-long tubes that help rapidly convert carbon dioxide into calcium carbonate, a solid mineral found in eggshells, the shells of various marine organisms, calcium supplements and cement. The micromotors have an outer polymer surface that holds the enzyme carbonic anhydrase, which speeds up the reaction between carbon dioxide and water to form bicarbonate. Calcium chloride, which is added to the water solutions, helps convert bicarbonate to calcium carbonate.
The fast and continuous motion of the micromotors in solution makes the micromotors extremely efficient at removing carbon dioxide from water, said researchers. The team explained that the micromotors’ autonomous movement induces efficient solution mixing, leading to faster carbon dioxide conversion. To fuel the micromotors in water, researchers added hydrogen peroxide, which reacts with the inner platinum surface of the micromotors to generate a stream of oxygen gas bubbles that propel the micromotors around. When released in water solutions containing as little as two to four percent hydrogen peroxide, the micromotors reached speeds of more than 100 micrometers per second.
However, the use of hydrogen peroxide as the micromotor fuel is a drawback because it is an extra additive and requires the use of expensive platinum materials to build the micromotors. As a next step, researchers are planning to make carbon-capturing micromotors that can be propelled by water.
“If the micromotors can use the environment as fuel, they will be more scalable, environmentally friendly and less expensive,” said Kaufmann.
Certainly nothing like this would be practical for a medical nanorobot, but as engineers work to improve these CO2 sequestration devices, they might invent improvements that would also be practical for medical nanorobots, or at least encourage thinking in that direction. And of course, a practical system to remove excess CO2 from the sea would be very nice, and is no doubt a lot nearer.
—James Lewis, PhD