Recently we noted an extensive review of the use of DNA scaffolds to orient molecules for molecular studies, as this capability could lead to organizing functional components for atomically precise manufacturing (APM). An excellent example of this capability of DNA scaffolds, published last year in Science [abstract] has been made available for open access: “Fluorescence enhancement at docking sites of DNA-directed self-assembled nanoantennas” (pdf). A press release from the Technische Universität Braunschweig (in German, translated by http://translate.google.com/):
Light on the nanoscale focus: Researchers present tiny lenses from nanoparticles and DNA
Conventional lenses may focus light only to a volume of about one femtoliters (10-15 liters), which corresponds to a cubic micron. This limitation is a result of diffraction, which is inherent in all conventional lenses, and prevents many applications in the field of nanotechnology. The research group led by Prof. Philip Tinnefeld, Institute of Physical and Theoretical Chemistry, Technical University of Braunschweig, now has developed a method, are produced in parallel with the millions of so-called nano-lenses of metallic nanoparticles and DNA. These nano-lenses allow us to investigate even single molecules up to one hundred times more precise.
In the emerging research field of nanophotonics scientists study the behavior of light in dimensions that are smaller than the wavelength of light. For example it is known that a laminate of two gold nanoparticles can focus light to a point, which is about a thousand times smaller than that of conventional lenses. Such a strong focus has great technological potential, for example for signal processing in optical computers, for ultra-sensitive detection in the diagnosis or also for biotechnological applications such as DNA sequencing. However, so far it has been a challenge to place gold nanoparticles having a size range of 80-100 nm at defined intervals and to bring the molecules to be analyzed in exactly the active “hot spot” between the particles.
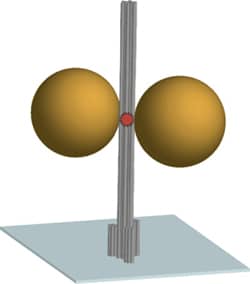
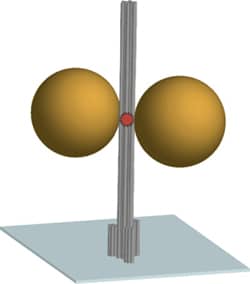
The diagram shows the DNA origami nanoscale pillar (gray) immobilized on a surface. Two gold nanoparticles with a diameter of 80-100 nm are used as an antenna and focusing the light in the hotspot between the nanoparticles. A fluorescent dye in the hotspot (red) is used as optically active source and reports the fluorescence enhancement.
This led the group of Prof. Philip Tinnefeld to search for a new approach to the development of nano-lenses. They found him in the so-called DNA origami technique. In this case genetic material of viruses used in this case, as the carrier material. DNA has a structure at the nano level the property can be folded into many possible forms. The Brunswick team of researchers has formed from this starting material, a nano-pillar. Specific molecules at the bottom of the column allows its upright position on a coverslip. These DNA nanocolumn serves as a scaffold to which the nanoparticles (gold particles in this case) have been attached. Now, tiny optical sources, such as fluorescent dye molecules, precisely placed between these particles. At that moment, the capacity of the nano-lens proves that the fluorescence of individual molecules increases by a factor of hundred.
Scientists are convinced that this new approach will have a major impact on various fields of research. Professor Philip Tinnefeld describes the range of possible applications: “Because the light is concentrated in the smallest volume in the range of Zeptolitern, we are able to examine individual objects with an improved signal and at higher concentrations. This is particularly important for biological applications, since many relevant processes such as DNA replication can proceed efficiently only at higher concentrations. In addition, fundamental physical aspects of the interaction of light and nanoparticles of research are accessible as optical sources are now specifically be placed in the focus of nano lens.”
The scaffolded DNA origami structure built in this article differs from most other applications in being a tall pillar held erect on a surface. The DNA pillar is 220 nm long by 15 nm in diameter, with three biotin-modified 6-helix bundles forming a 30 nm-diameter base to immobilize the pillar on a surface coated with a biotin-binding protein. Additional DNA strands capture two gold nanoparticles and a dye molecule at specific positions on the pillar. Two 80-nm diameter gold particles (20- to 100-nm in different experiments) were rigidly positioned 23 nm apart using three DNA strands per particle. The observed fluorescence enhancement (up to 117 fold) was strongly dependent on the relative placement of the dye molecule and nanoparticles. Perhaps the next step toward APM is to determine if DNA origami-mediated precise placement of molecular reaction components can improve precision control of complex reaction sequences the way that precision placement of components improves nanophotonic applications.
—James Lewis, PhD