The most fundamental dimension in the transition from current nanotechnology, which is mostly materials science and simple devices, to the advanced nanotechnology of productive nanosystems and atomically precise manufacturing will be the dimension of greater control of the structure of matter leading to atomic precision. But another important dimension is imbuing matter with intelligence. Ultimately that intelligence will be embodied in control by atomically precise digital computers, but steps toward that goal are resulting from nanocomposites that combine a sensing and an effecting function. A hat tip to ScienceDaily for reprinting this Lawrence Berkeley National Laboratory news release written by Alison Hatt “Raising the IQ of Smart Windows“:
Researchers at the U.S. Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) have designed a new material to make smart windows even smarter. The material is a thin coating of nanocrystals embedded in glass that can dynamically modify sunlight as it passes through a window. Unlike existing technologies, the coating provides selective control over visible light and heat-producing near-infrared (NIR) light, so windows can maximize both energy savings and occupant comfort in a wide range of climates.
“In the US, we spend about a quarter of our total energy on lighting, heating and cooling our buildings,” says Delia Milliron, a chemist at Berkeley Lab’s Molecular Foundry who led this research. “When used as a window coating, our new material can have a major impact on building energy efficiency.”
Milliron is corresponding author on a paper describing the results the journal Nature [abstract]. …
Milliron’s research group is already well known for their smart-window technology that blocks NIR without blocking visible light. The technology hinges on an electrochromic effect, where a small jolt of electricity switches the material between NIR-transmitting and NIR-blocking states. This new work takes their approach to the next level by providing independent control over both visible and NIR light. The innovation was recently recognized with a 2013 R&D 100 Award and the researchers are in the early stages of commercializing their technology.
Independent control over NIR light means that occupants can have natural lighting indoors without unwanted thermal gain, reducing the need for both air-conditioning and artificial lighting. The same window can also be switched to a dark mode, blocking both light and heat, or to a bright, fully transparent mode.
“We’re very excited about the combination of unique optical function with the low-cost and environmentally friendly processing technique,” said [Anna] Llordés, a project scientist working with Milliron. “That’s what turns this ‘universal smart window’ concept into a promising competitive technology.”
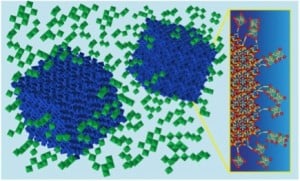
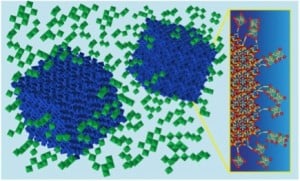
At the heart of their technology is a new “designer” electrochromic material, made from nanocrystals of indium tin oxide embedded in a glassy matrix of niobium oxide. The resulting composite material combines two distinct functionalities—one providing control over visible light and the other, control over NIR—but it is more than the sum of its parts. The researchers found a synergistic interaction in the tiny region where glassy matrix meets nanocrystal that increases the potency of the electrochromic effect, which means they can use thinner coatings without compromising performance. The key is that the way atoms connect across the nanocrystal-glass interface causes a structural rearrangement in the glass matrix. The interaction opens up space inside the glass, allowing charge to move in and out more readily. Beyond electrochromic windows, this discovery suggests new opportunities for battery materials where transport of ions through electrodes can be a challenge. …
The key to this work appears to be a process for covalently bonding nanocrystals into an amorphous material so that the structure of the amorphous material can be manipulated.
—James Lewis, PhD