More complex nanoparticle structures provide the opportunity to integrate multiple functions, enabling nanotechnology to play a larger role in industrial processes. A hat tip to Nanowerk for reprinting this Rice University News release “Tiny octopods catalyze bright ideas“:
Nanoscale octopods that do double duty as catalysts and plasmonic sensors are lighting a path toward more efficient industrial processes, according to a Rice University scientist.
Catalysts are substances that speed up chemical reactions and are essential to many industries, including petroleum, food processing and pharmaceuticals. Common catalysts include palladium and platinum, both found in cars’ catalytic converters. Plasmons are waves of electrons that oscillate in particles, usually metallic, when excited by light. Plasmonic metals like gold and silver can be used as sensors in biological applications and for chemical detection, among others.
Plasmonic materials are not the best catalysts, and catalysts are typically very poor for plasmonics. But combining them in the right way shows promise for industrial and scientific applications, said Emilie Ringe, a Rice assistant professor of materials science and nanoengineering and of chemistry who led the study that appears in Scientific Reports [“Resonances of nanoparticles with poor plasmonic metal tips“, open access].
“Plasmonic particles are magnets for light,” said Ringe, who worked on the project with colleagues in the U.S., the United Kingdom and Germany. “They couple with light and create big electric fields that can drive chemical processes. By combining these electric fields with a catalytic surface, we could further push chemical reactions. That’s why we’re studying how palladium and gold can be incorporated together.”
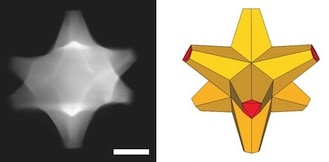
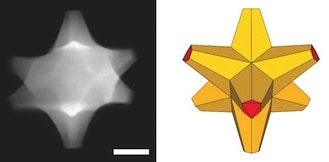
The researchers created eight-armed specks of gold and coated them with a gold-palladium alloy. The octopods proved to be efficient catalysts and sensors.
“If you simply mix gold and palladium, you may end up with a bad plasmonic material and a pretty bad catalyst, because palladium does not attract light like gold does,” Ringe said. “But our particles have gold cores with palladium at the tips, so they retain their plasmonic properties and the surfaces are catalytic.”
Just as important, Ringe said, the team established characterization techniques that will allow scientists to tune application-specific alloys that report on their catalytic activity in real time.
The researchers analyzed octopods with a variety of instruments, including Rice’s new Titan Themis microscope, one of the most powerful electron microscopes in the nation. “We confirmed that even though we put palladium on a particle, it’s still capable of doing everything that a similar gold shape would do. That’s really a big deal,” she said.
“If you shine a light on these nanoparticles, it creates strong electric fields. Those fields enhance the catalysis, but they also report on the catalysis and the molecules present at the surface of the particles,” Ringe said.
The researchers used electron energy loss spectroscopy, cathodoluminescence and energy dispersive X-ray spectroscopy to make 3-D maps of the electric fields produced by exciting the plasmons. They found that strong fields were produced at the palladium-rich tips, where plasmons were the least likely to be excited.
Ringe expects further research will produce multifunctional nanoparticles in a variety of shapes that can be greatly refined for applications. Her own Rice lab is working on a metal catalyst to turn inert petroleum derivatives into backbone molecules for novel drugs.
These results show that the relationship between structure and function is likely to prove complex. Here, gold alloys containing the poor plasmonic metal palladium nevertheless produced strong plasmon resonances in the palladium-rich octopod tips. Learning how to design composite nanoparticles to produce such effects may be a challenge, but the results of this initial work look promising, and well worth the effort.
—James Lewis, PhD