Summary
In this session, James Kirkland from Mayo Clinic gave a comprehensive overview of the current state of research about senescent cells and senolytic clinical trials. He provided nuanced insights into how senescent cells become senescent, how they work, what kind of functions can they play, and much more. He also went through 15 clinical trials that are currently testing senolytics on different indications and provided explanations and motivations behind the trials and what are the studies already in process finding, or discovered recently.
Presenters

James Kirkland, Mayo Clinic
James L. Kirkland, M.D., Ph.D., is Director of the Robert and Arlene Kogod Center on Aging at Mayo Clinic and Noaber Foundation Professor of Aging Research. Dr. Kirkland’s research is on the contribution of fundamental aging processes, particularly cellular senescence, to age-related diseases and development of agents and strategies for targeting fundamental aging mechanisms to treat age-related chronic diseases and…
Presentation: Clinical Trials & Senolytics
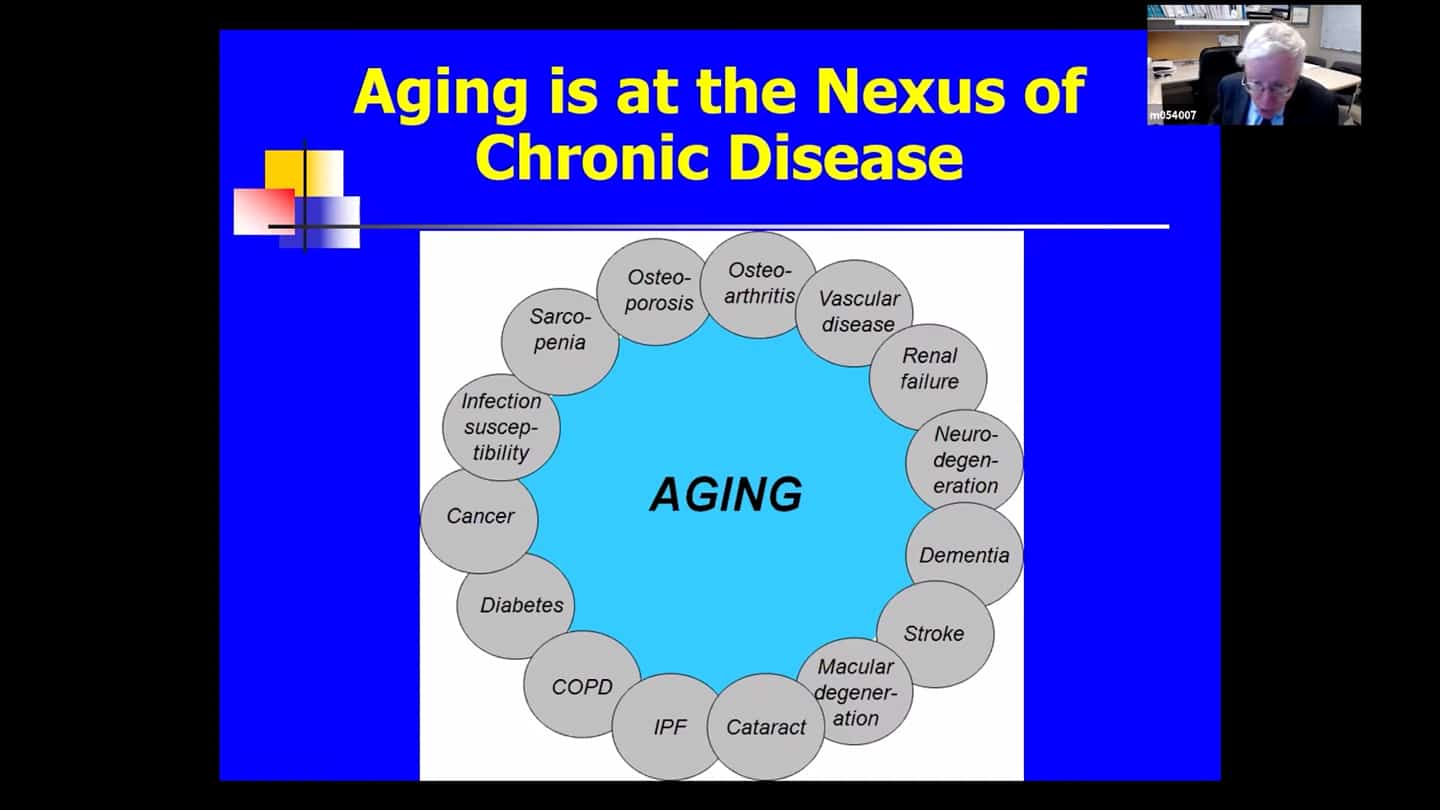
- Chronological age is by far the largest predictor of most chronic diseases that bulk for morbidity, mortality and health expenditures and is also connected to geriatric syndromes like frailty, age-related muscle impairment, malcognitive impairment as well as phenotypes that we see in older individuals, that is how they look and their characteristics and decreased physical resilience or ability to recover after surgery or infection. These aging processes begin at the time of conception.
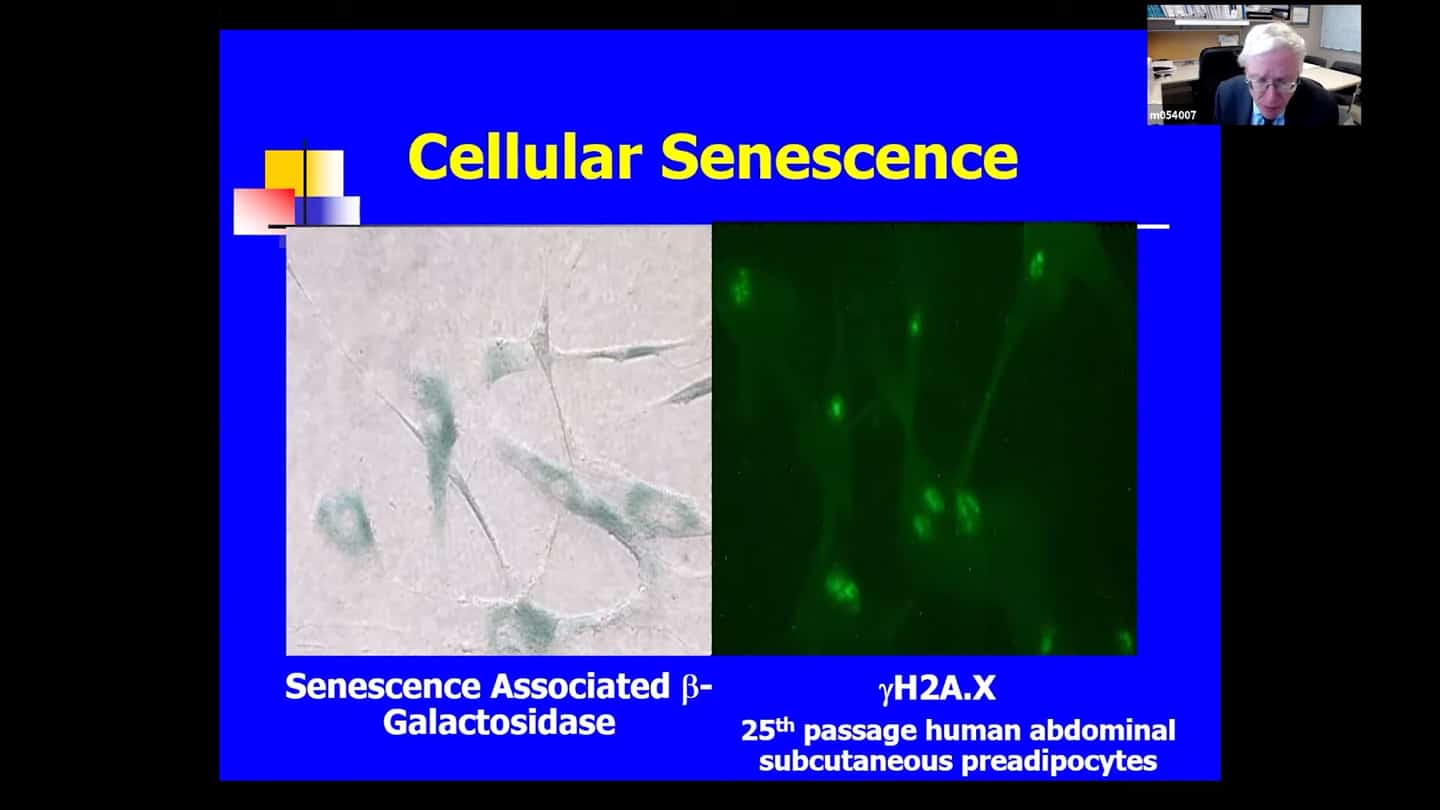
- Fundamental aging mechanisms can develop at sites of age-related diseases anywhere from early development to later life. Senescent cells are cells that have lost replicative potential and are essentially replicatively arrested, but they are viable. Some senescent cells are very metabolically active and have increased protein production, and they are hard to kill, resistant to apoptosis, programmed cell death, and are normally only removed by the immune system.

- There are no complete and sensitive markers of senescent cells, they accumulate at sites of age related and chronic diseases, and accumulate in healthier older individuals across multiple tissues at older life. But they can appear in any invertebrate at any point from conception on. Senescent cells in the placenta for example are responsible for producing factors driving the baby through the birth canal. Senescent cells clear up tissue damage after wounding. They are important during fetal development, and they act like a defense system against cancers – senescent cells will form as a response to stimuli that drive cells into cancer. If they appear in the middle of a cancer for example, they slow down the growth of the cancer. But also roughly 30-70% of senescent cells have a senescence associated secretory phenotype, where they produce factors that kill cells around them.
- So you don’t wanna interfere with the ability to generate senescent cells, but once they are there, and are not cleared by the immune system and reach a certain threshold, they start causing problems.
- Senescent cells have been found at sites of multiple chronic diseases, like in fat tissue in diabetes or the pancreas in diabetes, the brain in Alzheimer’s disease. They accumulate with advancing age, the rate of accumulation is slowed by intervention such as caloric restriction that are associated with increased healthspan, that was published in 2014, which prompted us to ask whether accumulation of senescent cells that have a SASP and are damaging, whether that is causal with respect to age-related phenotypes and geriatric syndromes and chronic diseases or it’s just associated. We began trying to develop ways to clear senescent cells, to ask if this was more than an association and was in fact causal relation. Eventually after trying multiple ways to kill senescent cells remembered that they survived despite the fact that the deleterious senescent cells kill cells around them, which lead us to hypothesise that they must have pathways that defend them against the things they’re using to kill the cells around them. Through proteomic and transcriptomic databases we found that they do have upregulated pro-survival pathways in a network that depends on the senescent cell type. And in some respect the other hypothesis that we used to develop senolytic drugs is that in some respect senescent cells are like cancer cells, and in fact many of them are cancer cells before they’ve become diving cancer cells. So we used bioinformatic and other approaches to develop senolytic drugs.

- Through that approach we also found that disabling some of the pro-survival networks will transiently allow the deleterious cells to kill themselves instead of damaging the cells around them. And that’s how senolytics act.
- Since new senescent cells take 10 days to 6 weeks to form, these senolytic agents can be administered in a hit and run manner – once or twice if there is an insult like radiation that’s not repeated, and do that periodically as new cells form. In those experimental situations the drugs can be given once every two weeks or once in a month and that has the same effects as giving these agents continuously. We are not trying to occupy a receptor or inhibit an enzyme, not looking for a steady state of the compound in blood, we’re trying to get rid of a cell type, allow these cells to kill themselves. Two to three hours of exposure is enough to do so. And then it takes ~18 hours for the cells to kill themselves. So that’s why the hit and run manner works sufficiently. That’s why we moved away from the old-fashioned single drug, single target, single disease approach to using combinations of agents in some cases or agents that work synergistically through multiple mechanisms to transiently disable these networks of pro-survival pathways, not just one target but networks of them that sometimes are redundant.
- And the view is that if the geroscience hypothesis is correct, we might be able to affect multiple age related disorders and chronic diseases that are associated with aging. So the strategy for developing these senolytic agents is much closer to developing antibiotics than drugs with single molecular targets.

- There are multiple animal models with various diseases and conditions in which intermittent senolytic administration appears to alleviate some of those conditions or delay or prevent them.
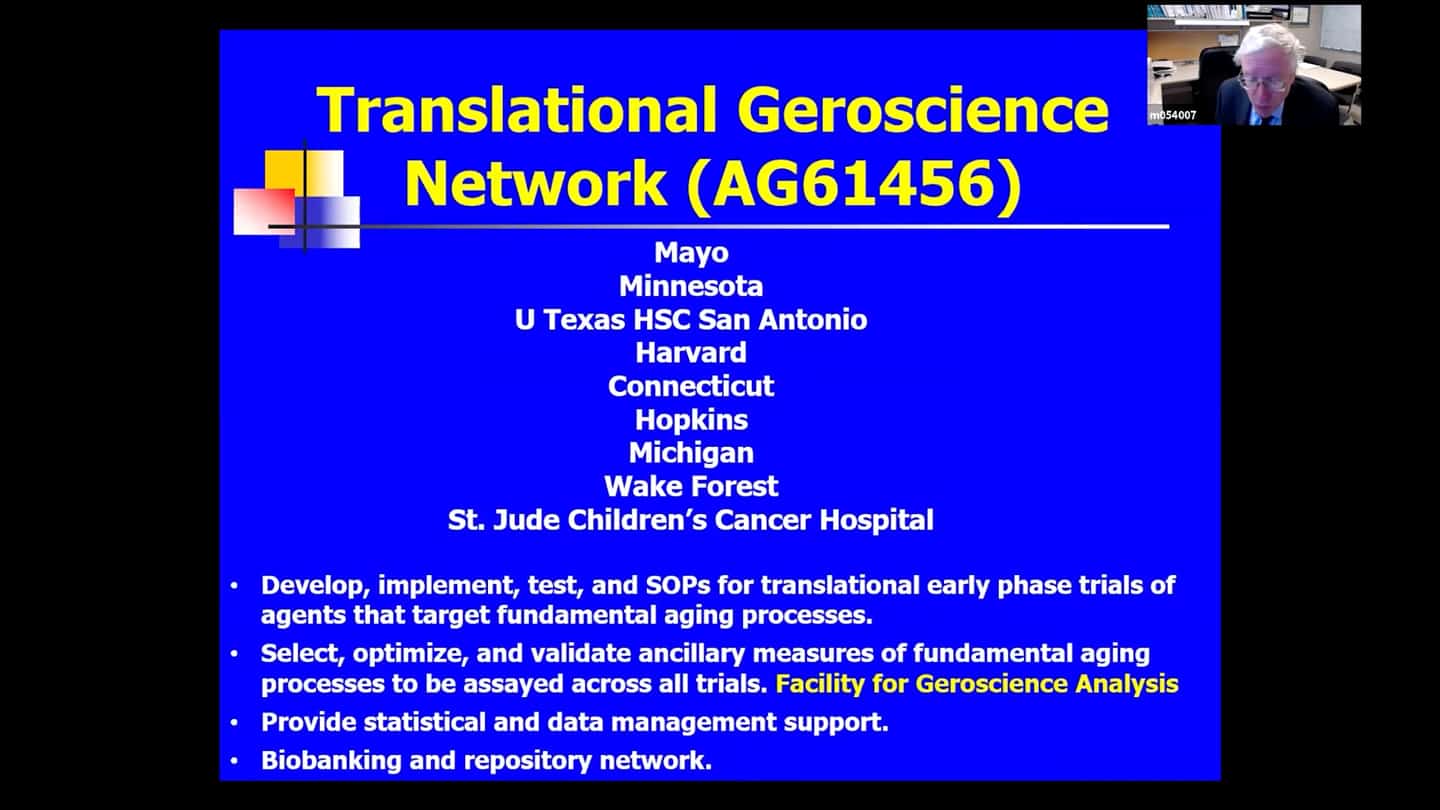
- We have a Translational Geroscience Network, it’s a consortium of 9 listed organizations, funded by the NIA. This network is there in order to help take drugs from the bench to the bedside in early stage clinical trials. One of the things we have through this network is a Facility of Geroscience Analysis where we can study samples (blood, urine, cheek swabs) that contain cells from across these clinical trials (not just senolytic trials, but also other agents that target fundamental aging processes, like rapamycin, metformin, and a list of others). Eventually we would like to combine some of these interventions to see if they are less than additive, additive, or synergistic, and we eventually want to do trials also with lifestyle interventions and with disease specific drugs. The question there is again whether combining these approaches result in a synergistic response over using just a drug that targets fundamental aging process alone vs targets of disease alone.
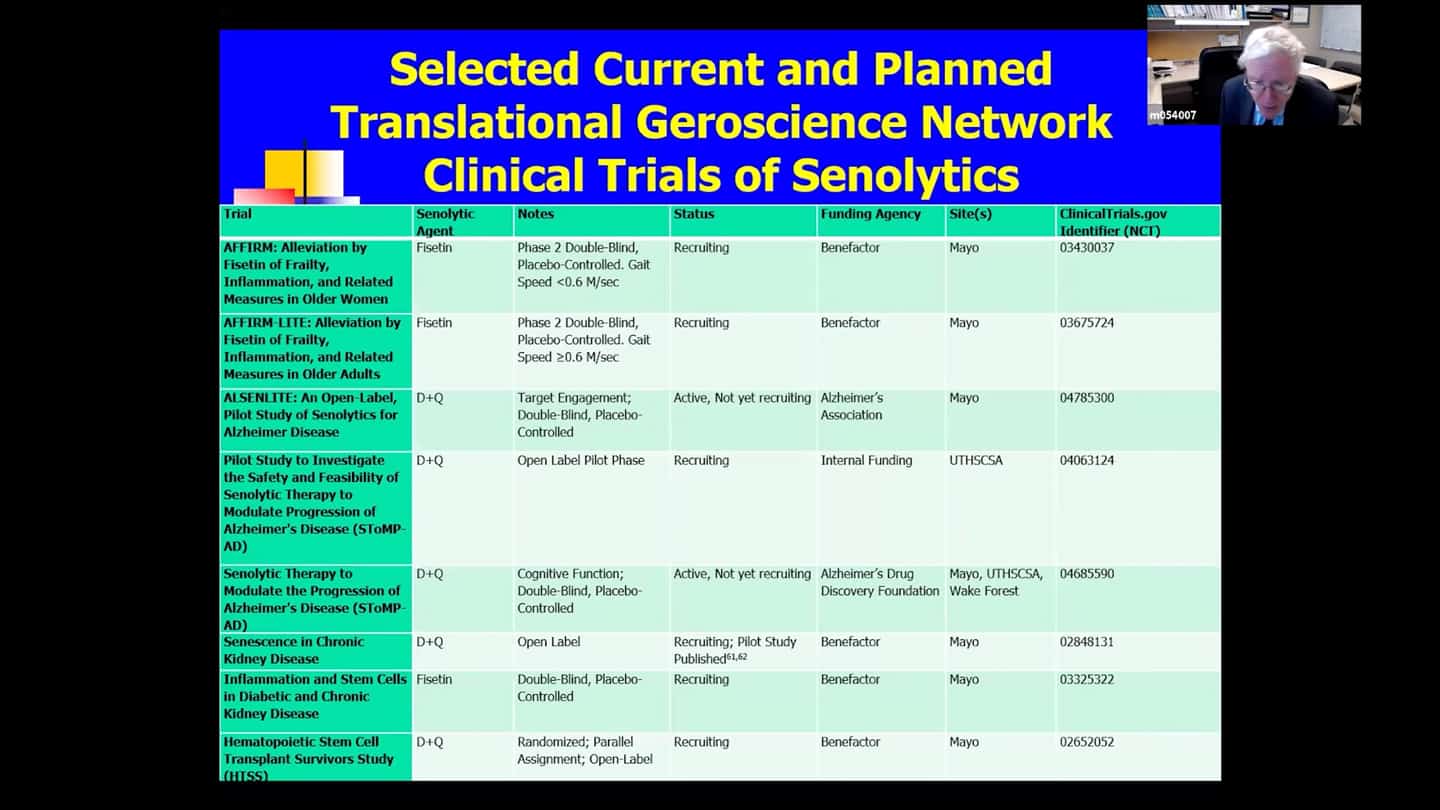
- In that network (there are other trials running outside of it as well), there are currently 15 senolytic clinical trials running, only a couple of early results yet, so we’ve got a long time to go before we see whether these drugs are safe and effective in humans. The earliest drugs chosen are Dasatanib and Quercetin, and also Fisetin. Quercetin alleviates the side effects of Dasatinib and acts on different classes of senescent cells, they have synergistic effects and are usually administered together. Fistein is present in strawberries, but you’d have to eat 15 pounds of strawberries in 5 minutes to get the dosage used in clinical trials and act as a senolytic agent in a hit and run manner. These agents were chosen because their side effect profiles are relatively well known and have short elimination half-lifes. We’re trying to use agents that we can expose the subjects briefly and then we want them out of the system. Couple of the trials are in frail and elderly subjects (AFFIRM trials), these are Fistein, placebo-controlled, both of them are about half-way through now. One of them is for elderly women with extreme frailty, with gait speed of less than 0.6m per second, which is a strong predictor that carries roughly 2 year 50% survival. So having a it’s probably a worse prognosis than having cancer.
- There are several trials just beginning:
- A pilot for Alzheimer’s disease (ALSENLITE) is beginning, they are just recruiting people now.
- There is a multicenter double-blind placebo-controlled trial for Alzheimer’s disease that’s about to begin (SToMP-AD).
- Trial for kidney disease.
- Trial looking at inflammation in stem cells in diabetes with preliminary results published.
- Trial with bone marrow transplants recipients, which is almost finished (HTSS). People who get a bone marrow transplant get a high dose of chemotherapy and radiation to kill their immune system before transplant, and that can unfortunately induce senescence. Turn out that a number of bone marrow transplant recipients after 3-5 years get into accelerated aging like state, so this one trial is testing whether clearing senescent cells might alleviate that accelerated aging like state where people get cognitive impairment, diabetes, second unrelated cancers, and atherosclerosis with heart attacks and strokes.
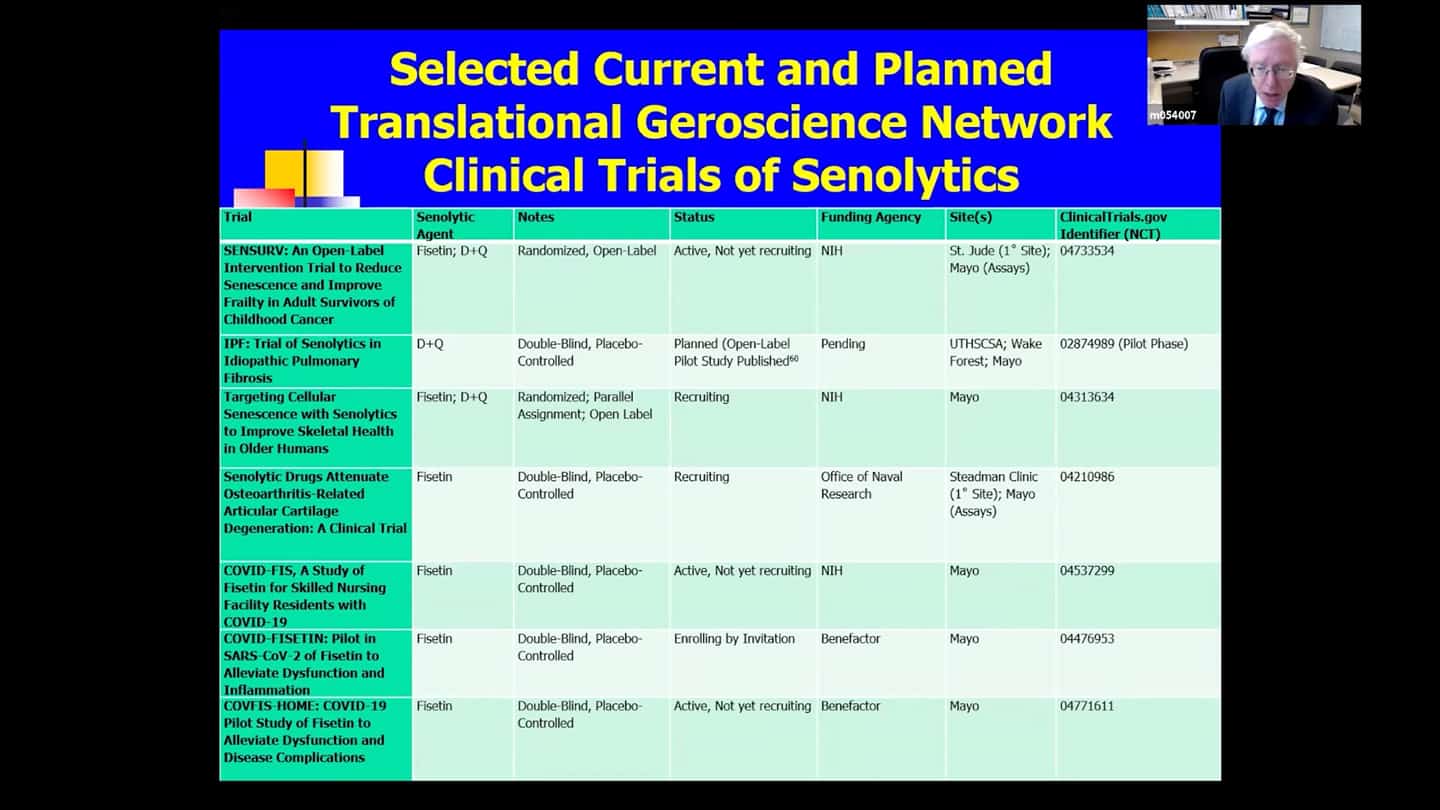
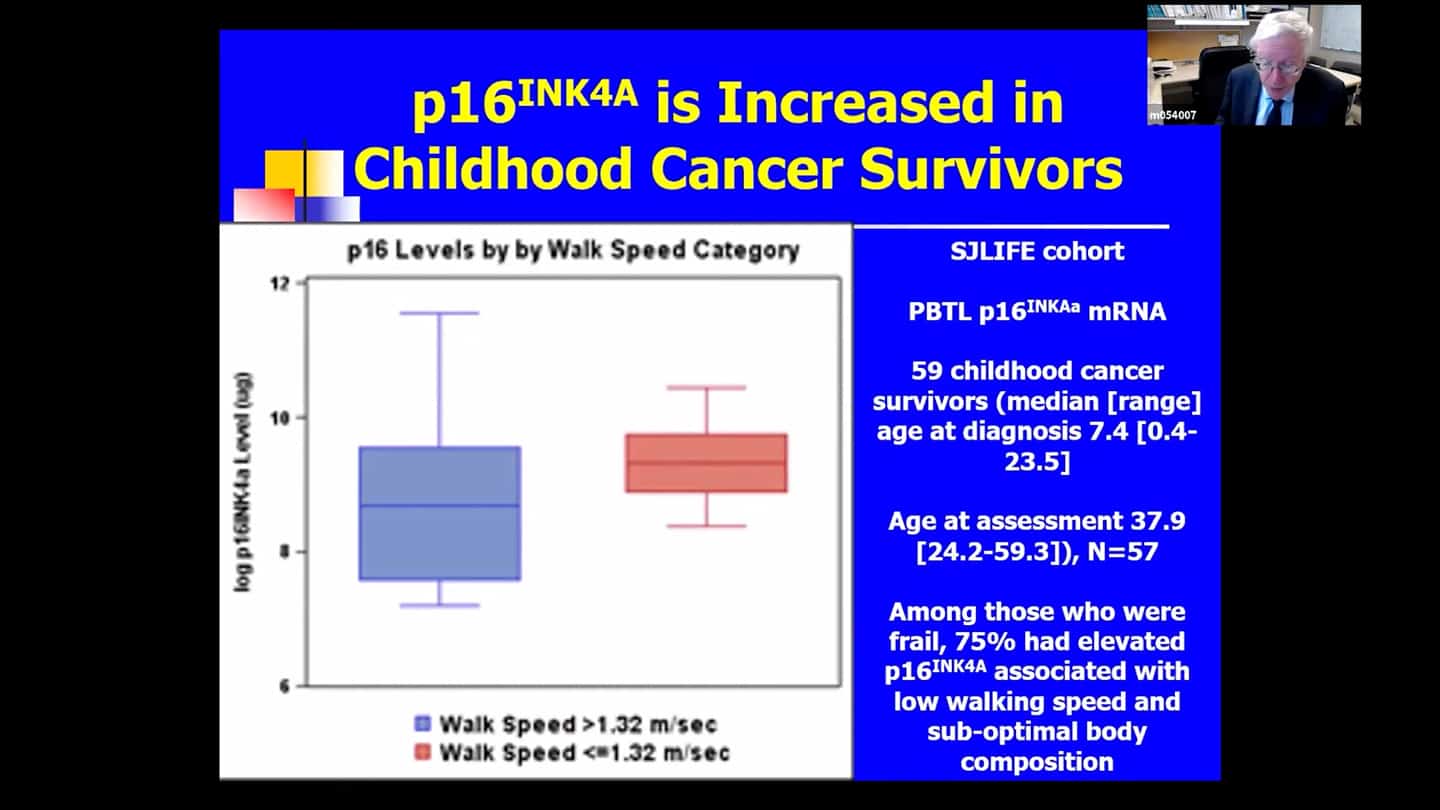
- Trial based on that same kind of logic at St. Jude, funded by the NIH (SENSURV). It’s comparing D+Q to F to placebo, it’s an open label randomized trial, which should be beginning in June, it’s funded and approved. And it’s looking at this accelerated aging like state again, which can develop in people in their thirties or forties if they were treated for cancers as children. It’s screening senescent and functional measures
- Trial looking at idiopathic pulmonary fibrosis (IPF). Preliminary results from an open label branch were already published. And there is another safety trial that is about to be published. Both are basically a leadup to a larger double-blind placebo-controlled trial.
- Trial looking at age related osteoporosis, it’s about half way through now, recruited more than half the subjects, funded by the NIH.
- Trial funded by the Office of Naval Research looking at osteoarthritis, a disorder linked to senescent cell accumulation in around the knee joints.
- Several trials are underway or beginning for coronavirus and it’s complications (COVID-FIS, COVID-FISETIN, COVFIS-HOME).
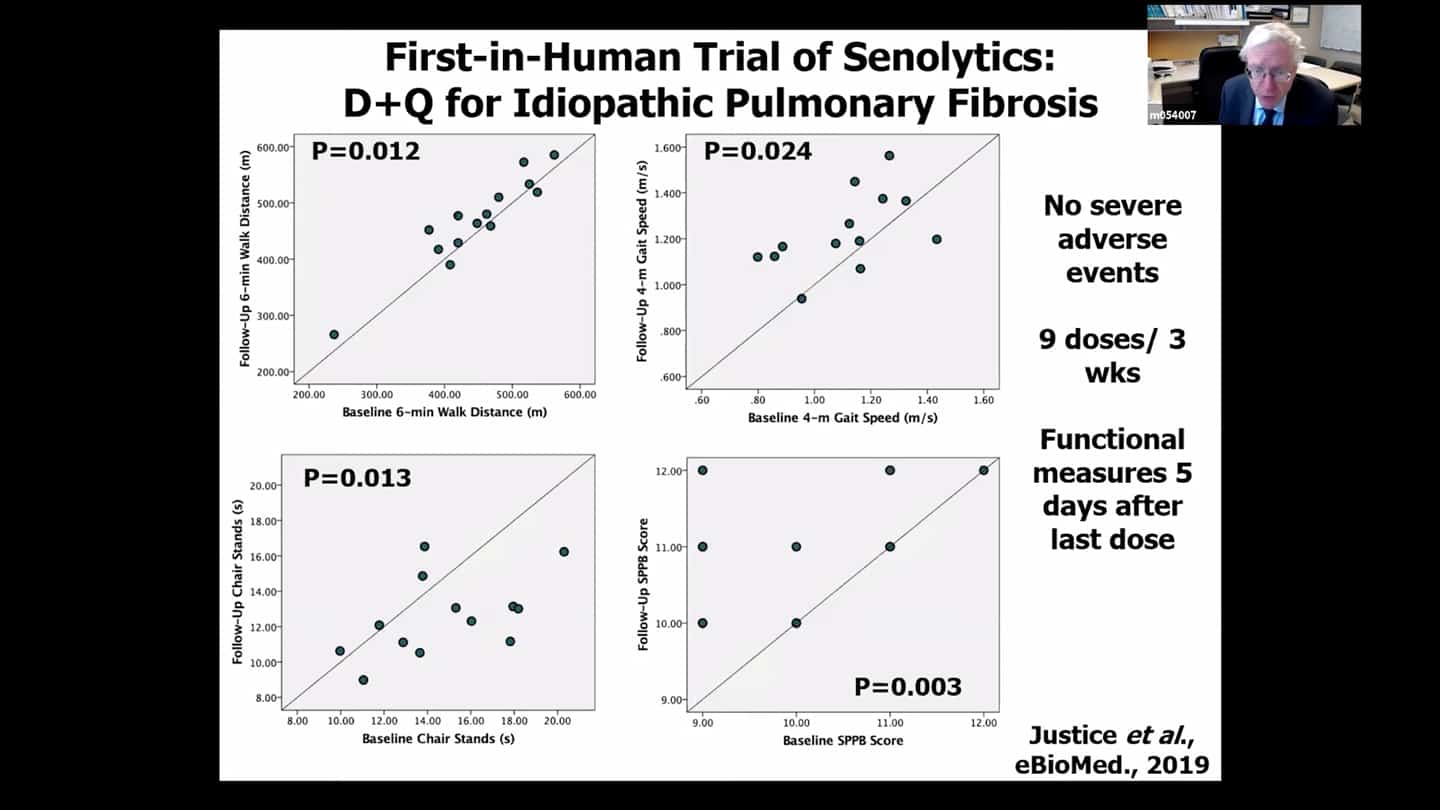
- Idiopathic pulmonary fibrosis (IPF) is usually fatal lung disease associated with senescent cell accumulation, in animal trials senolytics seem to alleviate the condition. Based on that an open label no placebo trial was done in humans. There seem to be improvements in functional measures in old people, but the trial design was not strong and needs to be done through double blind placebo controlled trial.
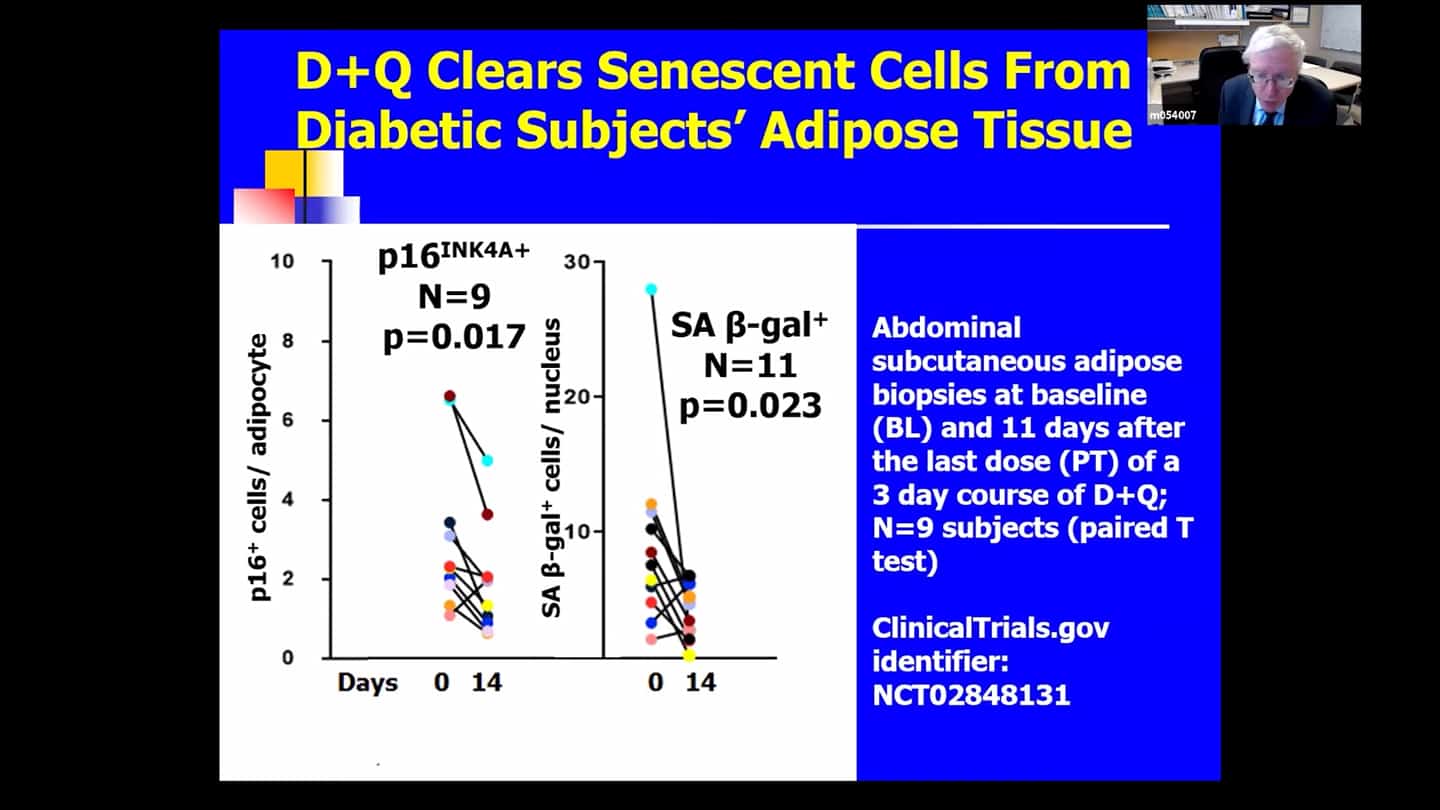
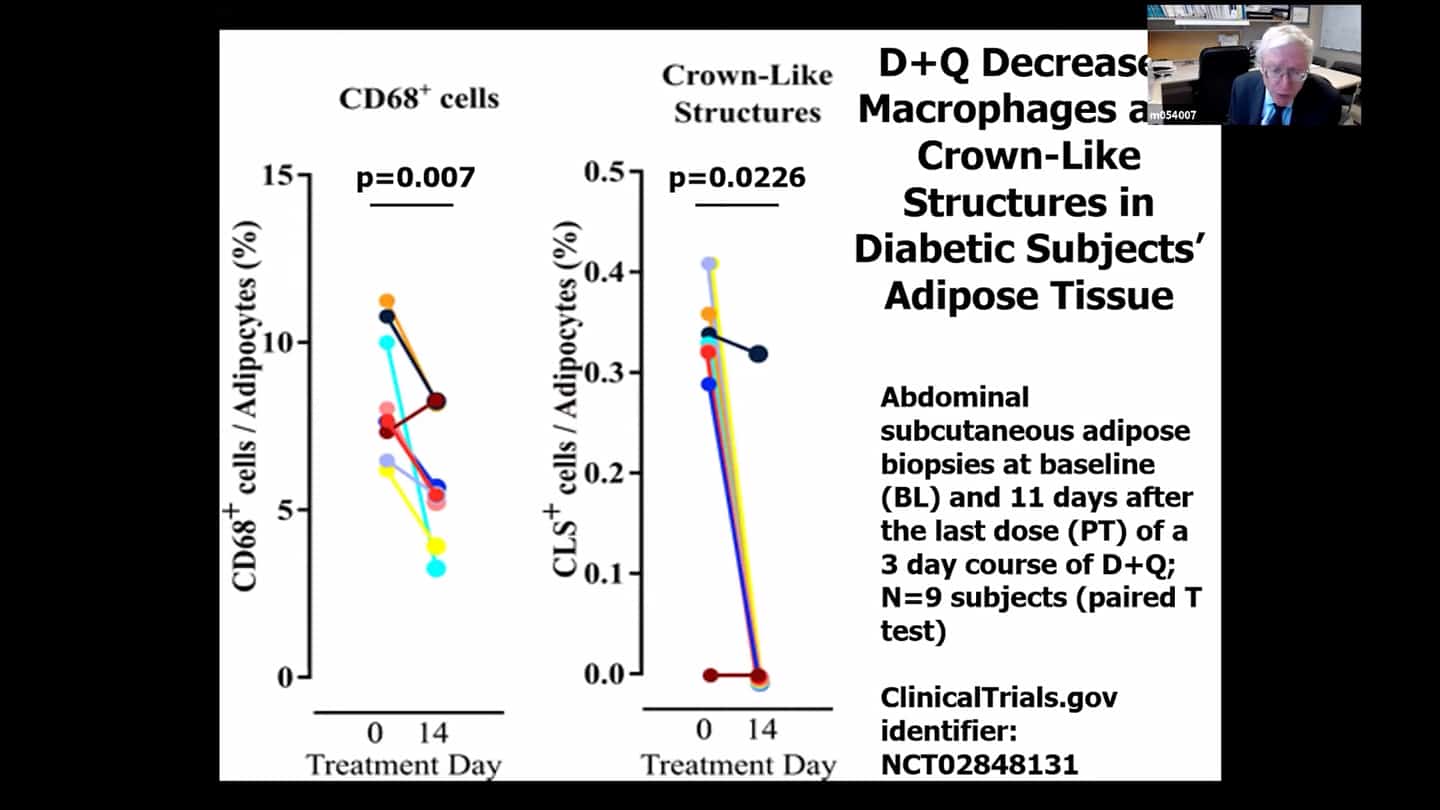
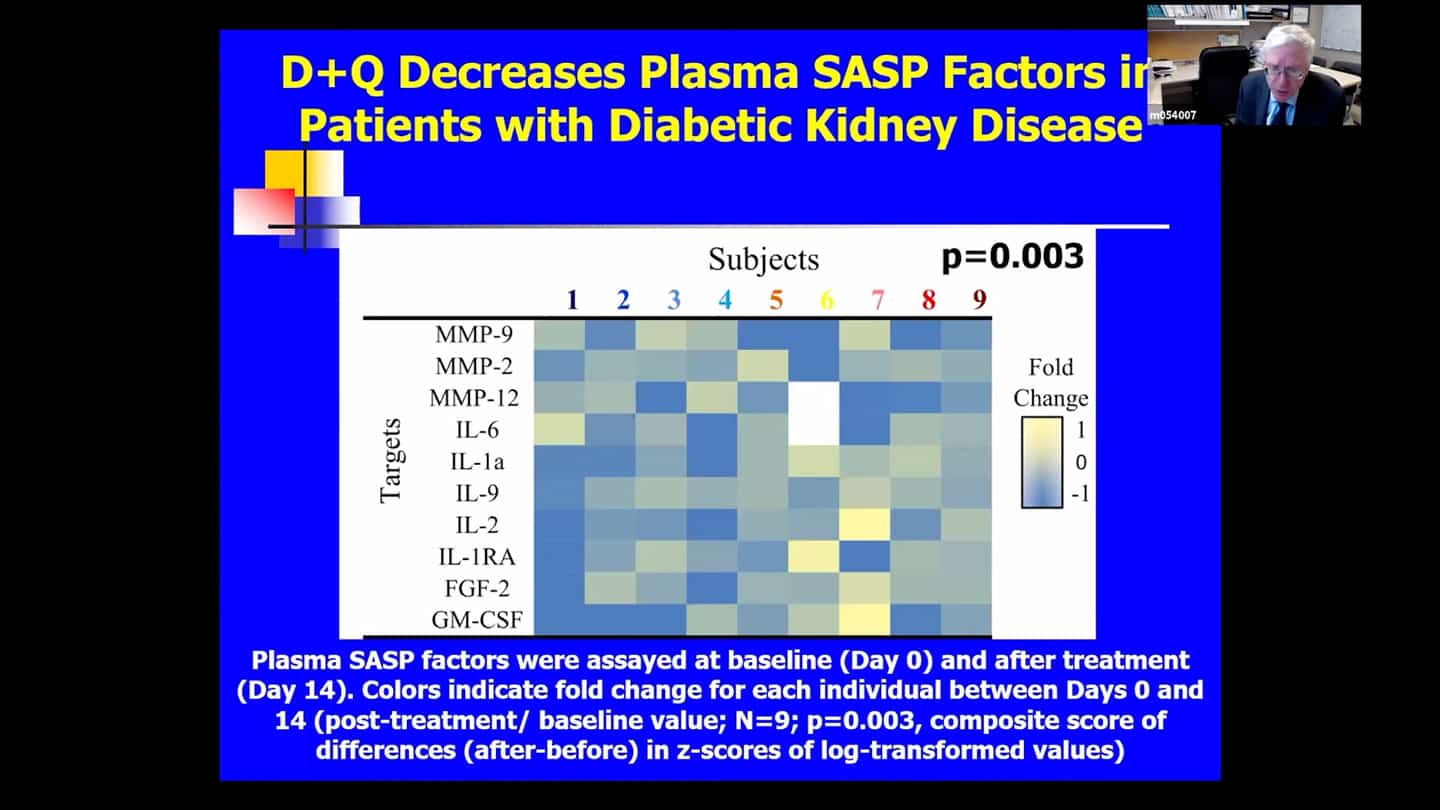
- There are no perfect markers of senescent cells, we usually need to use multiple measures and markers to be sure and convince ourselves that it really had an effect in clearing senescent cells.
- We can’t follow a single marker, so we’re looking at over 100 measures in the blood and are using a composite marker. Some of the markers are proteins in the blood, some of them related to DNA and RNA that are in the blood, cerebral spinal fluid or other body fluids. We measure a number of markers within cells directly, we look at methylation clocks, and all kinds of other things. Senescent cell burden seems to affect other pillars of aging and vice versa, so if we target one, we see effects in others as well most of the time.


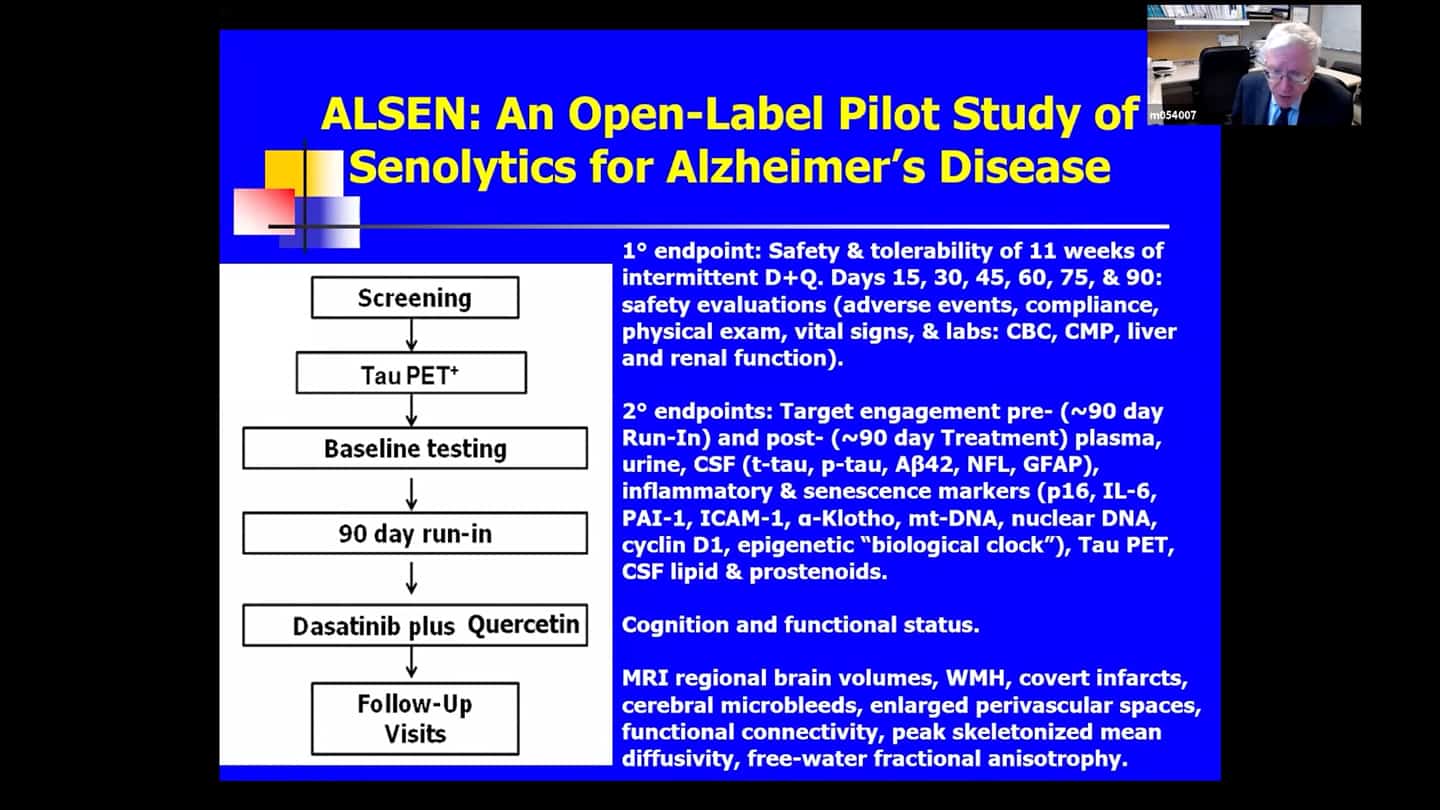
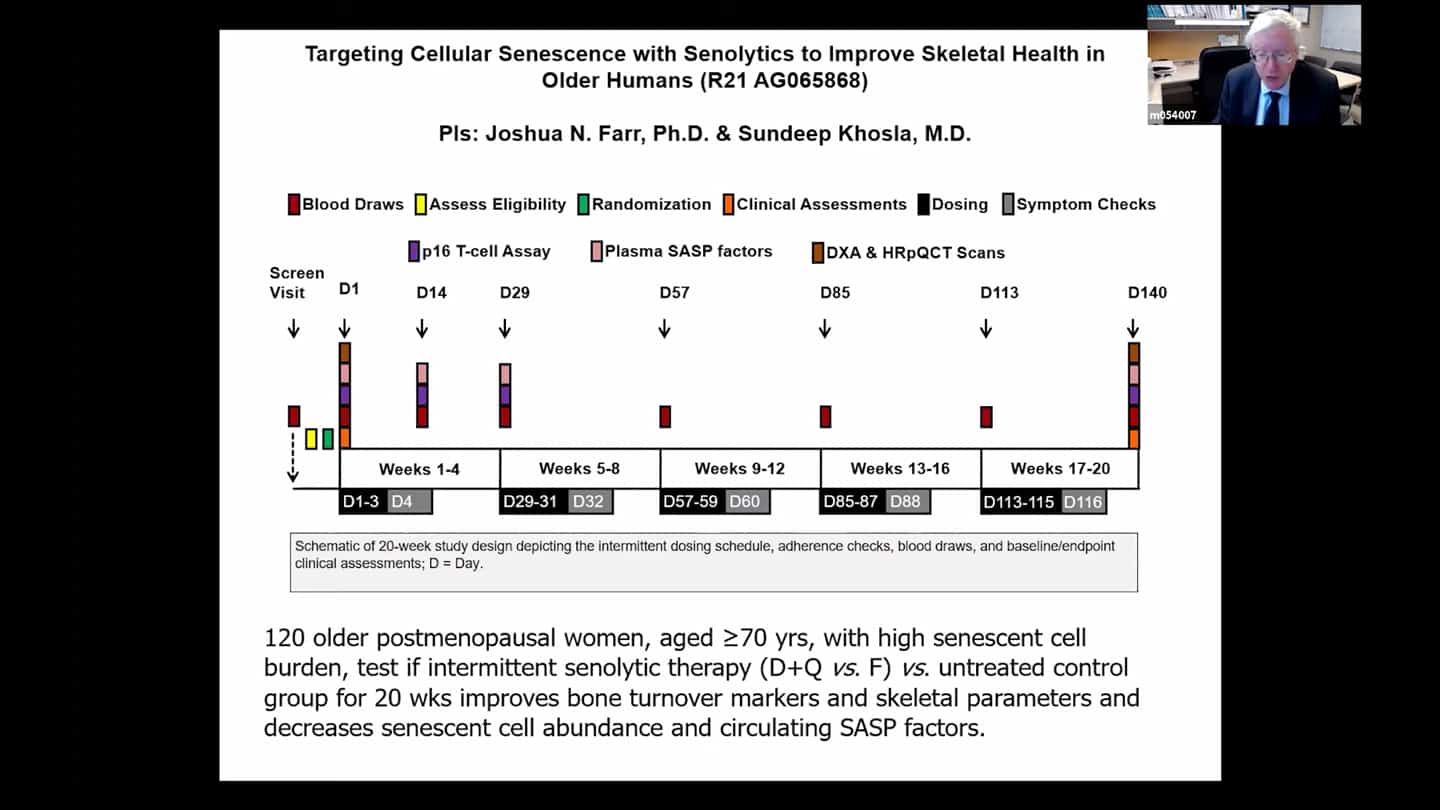
- Safety trial. We don’t know whether Q + D is safe, so we need to run a trial on it, so we know how big placebo controlled trials are going to have to be.

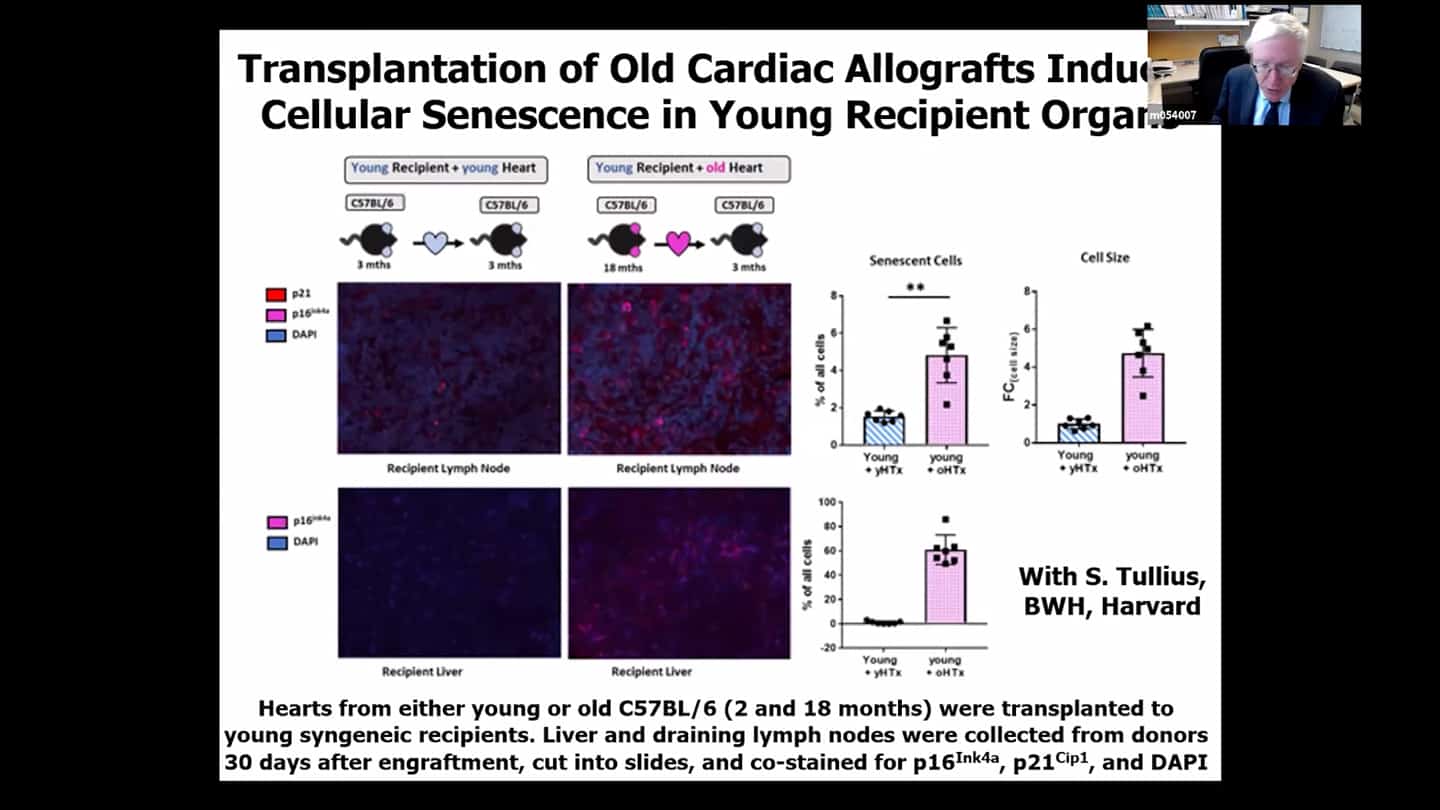
- Transplanting organs from the old to young mice resulted in faster accumulation of senescent cells in the young mice, basically a spread of senescence from the old organ to the young individuals own organs. This isn’t because of migration of the cells, but the recipient’s own cells start to become senescent. Transplant surgeons have known that organs from older donors do not work very well, and that’s why we are throwing 35000 kidneys a year from old donors.
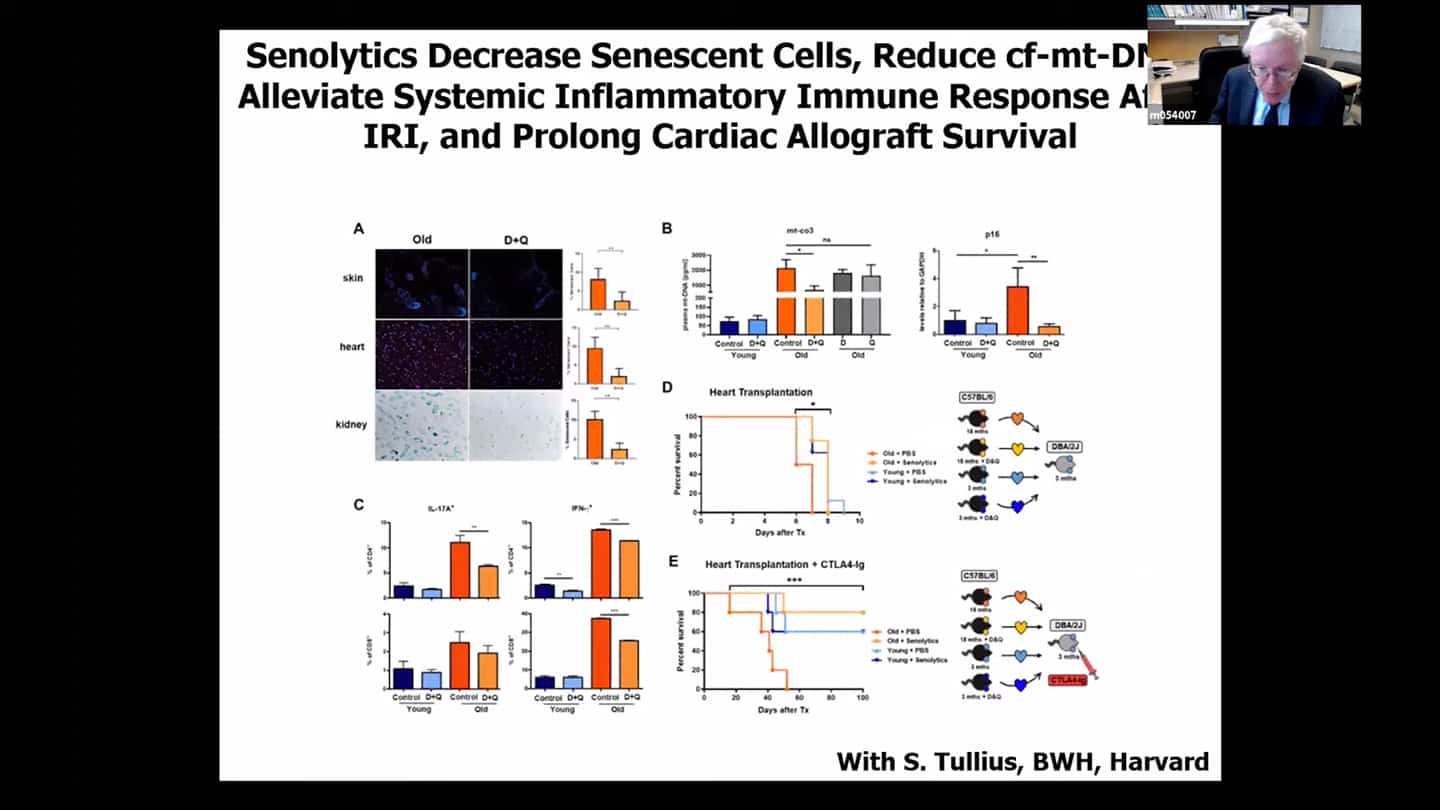
- In these heart transplanted animals, if we treat the donor or the recipient with senolytics, we are able to reduce the spread of senescence and are able to increase the survival of the recipient to the same level we see with recipients with hearts from younger individuals. So we actually get very good survival in the animal models.

- Which led to this early phase trial looking at using senolytics while the kidneys are on the perfusion machines, so we can utilize the organs from older donors. Similar thing is happening with liver transplants





- We don’t know whether senolytics work in humans, they could be really dangerous, senescence is a very fundamental process, we don’t know the downsides of clearing senescent cells, we’ve done a lot of research in animals to be as safe as possible. Right now, the only place where senolytics should be used is in carefully controlled clinical trials, where we use these agents in serious cases where we know that senescent cells are causal links. We don’t do trials in healthy people where we would need to wait 10-20 years for a result. And to speed things up even more, we do a lot of those trials in parallel.

Q&A
There are a lot of functions and properties and benefits of senescent cells that you went over in this presentation, at least in some cases. Is senescent really the right word? And how is it defined, is it just that they stop replicating and beyond that there are a lot of different possibilities of what they are doing – both good and bad?
- The definition of senescent cells is basically entry of a cell into essentially a cell cycle arrest, that’s irreversible unless there are further mutations or things that happen to the cell. It’s a cell fate, like replication, differentiation, apoptosis or necrosis. It’s the fifth cell fate. So any cell can become senescent. They can become senescent in response to damage signals, repeated replication, problems with replication, with intracellular organelles, in response to infectious pathogens, drugs, radiation, or other kinds of damaging insults. Senescence is basically defined as essentially irreversible loss of capacity to divide. It takes 10 days to 6 weeks for a cell to become fully senescent, so it is a slower process than other cell fates. Some but not all senescent cells can spread senescence and interfere with stem and progenitor cell functions, damage tissues and can produce a range of signals that detract, activate, and anchor immune cells. So this is a cell fate, a necessary process you don’t wanna interfere with. But if they become senescent and they persist and reach a threshold above which they spread faster than they are cleared out by the immune system, they start to take off and systemic problems can develop. There are probably two types of senescent cells at least, the nature of senescent cells depends on the nature of the starting cell type, what induced the senescence and how long the cell has been senescent. But 30-70% (so called deleterious senescent cells) produce damaging factors. And the rest do not produce damaging factors (so called helper senescent cells) – they are important for wound healing and so forth.
You said that “mitochondrial DNA is released from senescent cells” – that sounds like a serious problem. What’s going on here? Is it purely maladaptive or is there some good reason for it? It looks like a serious failure inside the cell system.
- One of the changes in senescent cells is that there is a really complex transcription factor cascade that enforces senescence, where literally thousands of genes expression gets changed. Mitochondrial dysfunction is a hallmark of senescence. Senescent cells are resistant to dying but they have a so-called “partial Warburg shift”, much like cancer cells, they depend on sugars rather than fatty acids. There’s a lot of turnover of mitochondrial DNA and changes in mitochondrial dynamics, so they release mitochondrial DNA and a lot of other non-coding nucleotides as well, like microRNAs that affect a lot of tissues as a great distance. Mitochondrial DNA is recognized as DAMP – danger associated molecular pattern protein – so other cells when they recognize that cells nearby are breaking down, they become senescent cells. So this is how senescence can spread even at a great distance. We’re working with some of the space agencies on space radiation research, we’ve got cells going up in 18 months on the Axiom flight.
Based on what you said about senescent cells and the Warburg effect – doesn’t that suggest that a really low carbohydrate diet might be senolytic?
- Not necessarily senolytics as such, but there are metabolic ways of getting at senescent cells and you do find that diet might impact senescent cell generation as it does with cancers – like caloric restriction which delays senescent cells accumulation and simultaneously improves healthspan. But we don’t know whether it’s the caloric restriction itself or carbohydrate restriction, so more work needs to be done. But these diets and lifestyle interventions are very hard to introduce in trials, especially in sick elderly people, compared to small molecule pills.
What the difference is between sensecent and terminal differentiation and how do senolytics achieve specificity?
- Terminal differentiation means that the cell has acquired a specialized cell fate and stops dividing. Senescent is in essence a terminally differentiated state, but some other terminally differentiated cells like neurons or cardiac myocytes can acquire senescence like state. That’s why it’s really hard to define senescence. It’s beginning to look like non-replicating cells can acquire a senescent like state as well, so the definition is tricky and there’s a debate and emerging view that terminally differentiated cells can acquire what looks like a senescence related fate.
Will you be doing mid-term interim analysis in the trials that are at half-way point?
- Some of them yes, most of them no, because of data safety and monitoring boards (these are FDA regulated trials).
Quercetin & Fisetin aren’t as strongly regulated as drugs so actual amounts of active ingredients vary from labeled amounts, sometimes a lot. (Companies like ConsumerLab make a business of testing these kinds of things.) How do you source for the trials & what do you do to ensure accurate dosing?
- We had to get a full IND for Fisetin. The minute you use a natural product to treat a disease, it is considered a drug. We had to register with the FDA as a drug. It took 2 years and 450 pages. We had to set up something called “Good Manufacturing Practices” manufacturing. We had to do stability testing, look for degradation products, test in multiple species, do safety and tolerability, we had to do all of that just like it’s a brand new drug before we were allowed to do clinical trials. We contract to a GMP manufacturer and then the Mayo Clinic mass spec facility monitors their product and we have to furnish to the FDA every couple of months safety and stability data.
Wound healing is important, so we don’t want to prevent cells from becoming senescent cells. In the case of wounds, we think most SnCs get cleaned up after. Do we know yet if that cleanup is less good with aging (maybe due to immunosenescence)? I.e., would we expect higher SnC burden in animals with higher rates of wounds?
- Some senescent cells produce platelet growth factor which helps wound healing. Those are the senescent cells that are not pro-inflammatory. Senolytics do not target those. Senolytics target 30-70% of cells that have SASP – senescence associated secretory phenotype – and are not active in wound healing. So we do not see the inhibition of wound healing with senolytics.
Are you saying that all the types of senescent cells that are involved in wound healing do not have SASP? Or just some of them?
- The SASP may be necessary early on to clear away dead tissue. But later on, it’s been shown in the animal models, if you clear cells (some of which are senescent) by targeting p16 expressing cells, you delay wound healing. But if you add back platelet growth factor, you restore wound healing. It turns out it’s the senescent cells that don’t have a SASP that produce that. So you do need them early on to help clear up the wound, you wouldn’t want to give these drugs right away, but in a chronic wound they may help.
So as we undergo immunosenescence and maybe the cleanup is imperfect, would we expect that animals that get higher rates of idiosyncratic repeated wounds will end up with a higher senescent cell burden?
- We’re not sure about that, but we see that pattern in other kinds of scenarios. There is what we call “stairway effect” – you have something that causes senescent cell burden, and then you get a bit better and you get back to baseline. Above a certain threshold, senescent cells start impeding immune system function and their own clearance.
You said monthly hit-and-run dosing is as good as continuous. Would you expect annual dosing to be effective?
- It depends on the rate of senescent cell formation, so it depends on the condition. If you blast a mouse with a one-time burst (like radiation), you just need to give a couple doses in the animal’s entire life to alleviate dysfunction. If you’ve got continuous high fat feeding, new senescent cells are generated at a particular rate, so it depends on the stimulus. It depends on the cell type that’s becoming senescent and a lot of other things. We’re only just beginning to get a handle on that. One of the things we use the blood test for is to try to figure out when senescent cells are coming back. We try to miniaturize that and make it as fast as possible so we can do in some of these trials intermittent blood testing like every few days and determine when there is a takeoff of senescent cells again so we can dose at that point.
How much is the senescence of several layers of cells in the liver an adaptive response to the aggressive environment and how much could senolytics interfere with this response?
- You don’t see much senescent cells in the liver in younger healthier individuals. You see them in conditions like primary sclerosing cholangitis, where they are very abundant and where they occur in one particular part of the liver. There is a clinical trial envisaged in that, so that will be coming along. In the case of hepatic steatosis where there is fat tissue that gets into the liver in the context of obesity and diabetes, there are different kinds of cells in the liver than in sclerosing cholangitis, so it’s a different set of cells in a different part of the liver that are affected. But you tend not to see many that we are able to detect in healthier individuals in the liver.
Are there any known negative interactions between the human immune system and senolytic therapy?
- It depends on the drug, there are probably a well over a 100 drugs now. Some drugs are very toxic, things like Noviclax for example. It is not an approved drug, it is being investigated, and it does have a lot of effects on the immune system. It mainly affects platelets but can cause unpredictable decreases in the white count, especially neutrophils. And after a while, there seems fairly severe neutropenia. While other senolytics don’t seem to have a big effect on immune cell numbers.
How much did you end up paying for that whole GMP for Fisetin work? (CMC and the other IND-enabling studies)
- Quite a lot, don’t know the exact numbers, we negotiated with different manufacturers, so it depends on the particular study. One thing we’re finding about Fisetin is that it’s pretty stable in our hands, we see that when we do mass spec on it. We were worried it would break down easily. In the right storage conditions, it is remarkably stable.
Will that data be available for that whole process with Fisetin – purification, stability testing, and all that kind of stuff?
- Yes, it will be, when we will be publishing some of the trials. We are developing a website about that particular trial with NIH, which should be coming online in the next week or so. It won’t have everything at the beginning, but eventually it will. Everything not considered business confidential will be available.
Do you have the clinical blood tests & expanded biochemistry tests from patients before and after administration of senolytics? Did you try any of the biological aging clocks trained on this data (e.g. Young.AI)?
- Yes, that’s all being done. But we don’t know yet, the data are just starting to come in. There is a group at Yale interested in the data, epigenetics processing is done at University of Minnesota, and then Morgan Levine and others at Yale are looking at the results. The data will eventually be public, because these are publicly funded trials.
You made a decision to go with and study systemic administration. Oral dosing, because of the signaling aspect, etc. Given the number of studies, any plans to try any local administration in any trials? (Unity’s failure could be due to locality, but could also be the wrong molecule.) (Unity’s failure could also be a poor choice of indication. Other indications with higher and maybe more temporary SnC burden may be better for local administration.)
- We find there is a spread of senescence in model organisms and based on early data in humans as well. Because of the spread of senescence throughout the organism, we’re concerned that local accumulation will be a harbinger of systemic problems, therefore systemic is the way. Also because of the problem of local administration and the many things it can mean when there is damaged tissue and a lot of blood supply to that tissue. And local administration spreads in the end systemically as well, so you could basically be giving at least intramuscular injection if not intravenous injection, when trying to administer something locally to a damaged tissue. Skin and inhaled agents are something different and might be worth looking into.
I suspect it doesn’t make sense to do local when you’re looking at the very very frail which those trials seem to be, but as you start to look into trials for younger healthier people possibly there are conditions where there’s a very local high senescence cell burden that hasn’t spread out too much yet?
- We’ve got some stuff emerging from Jon Hopkins that in fact in some younger people with local accumulation of senescent cells we see that they’re everywhere if we look.
What can the group do to help with your work? What would be helpful?
- We’ve got a lot of help from the government and foundations. What’s very important for people is to get the message out to not take the drugs in an uncontrolled way, because we don’t know whether these drugs are safe and effective. I give it maybe a 50:50 chance. We’re doing the trials because we don’t know if they’re going to work. As a physician I am very concerned with the Hippocratic oath and the first principle – “first, do no harm”. It’s a very fundamental process we are playing with, so we’ll have to see what happens and prefer conservative approach at this point. But we’re trying to move quickly because we feel it’s important to know one way or the other, but I worry.
Seminar summary by Bolek Kerous.
