Presenter

Sergiy Velychko
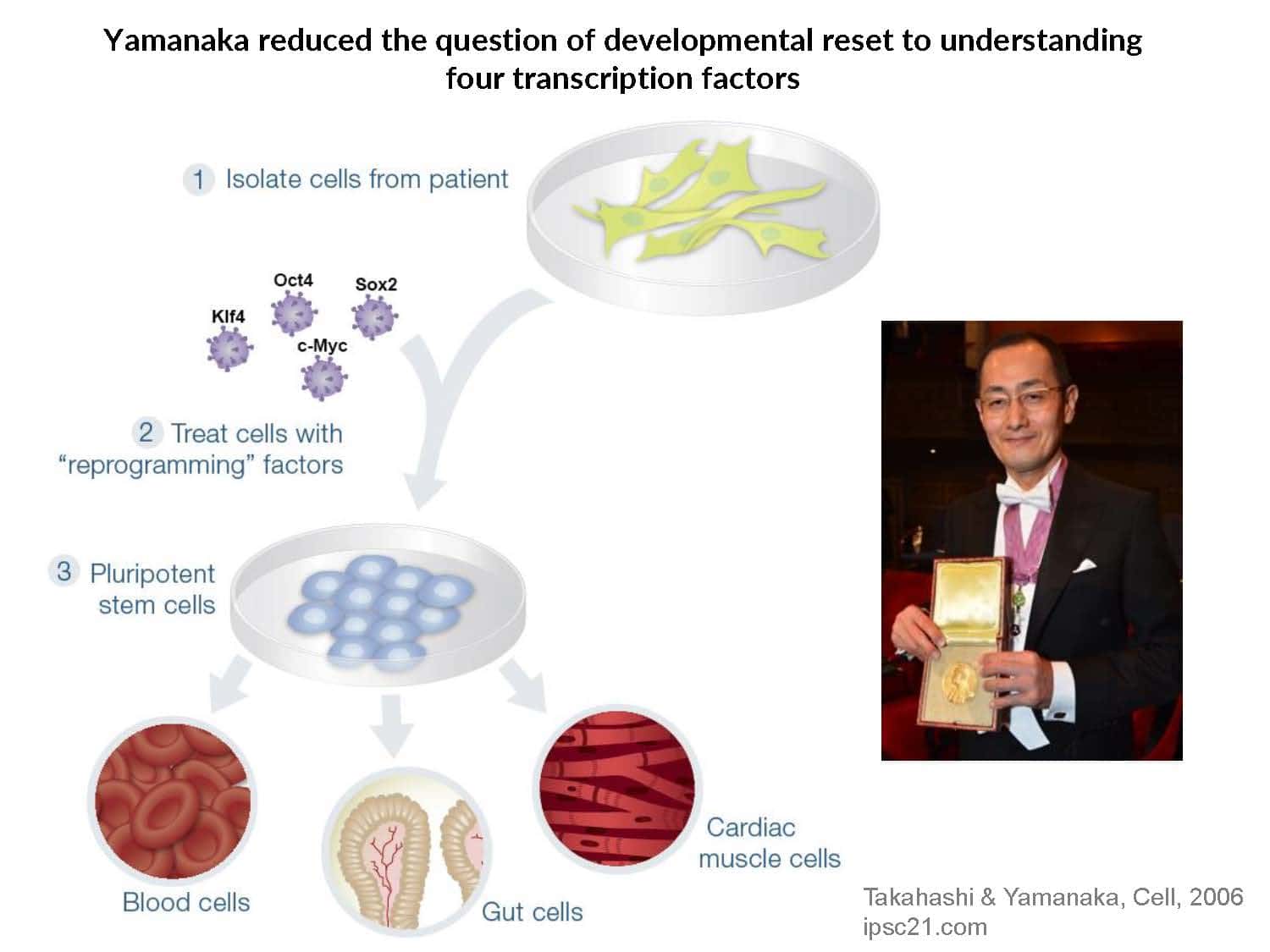
Sergiy Velychko is a postdoc in the lab of George Church at Harvard Medical School, focusing on synthetic developmental biology. He studied Biology and Biotechnology at Kyiv National University, then pursued a Master's degree in Molecular Bioengineering from the Technical University of Dresden. His thesis focused on the genetic engineering of human iPSCs for disease correction. Sergiy continued his research in the department of Hans Schöler at the Max Planck Institute in Münster, where he defended his PhD on Oct4’s role in pluripotency. Sergiy's cover article in Cell Stem Cell was recognized with the “Best Publication of the Year Award” from the German Stem Cell Network. His recent contributions include the development of an enhanced Yamanaka factor, super-SOX, and elucidating the mechanism of naïve pluripotency.
Summary:
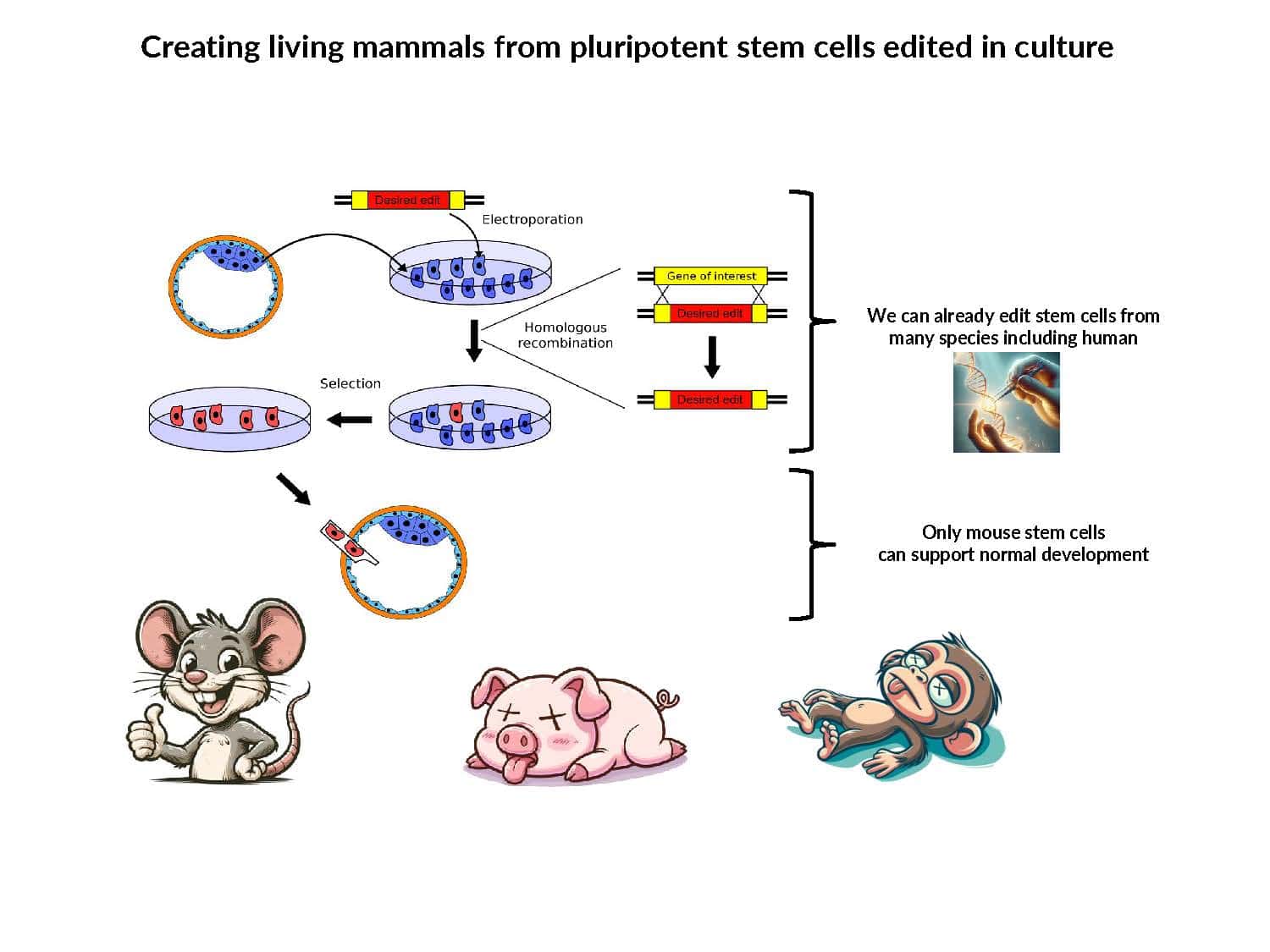
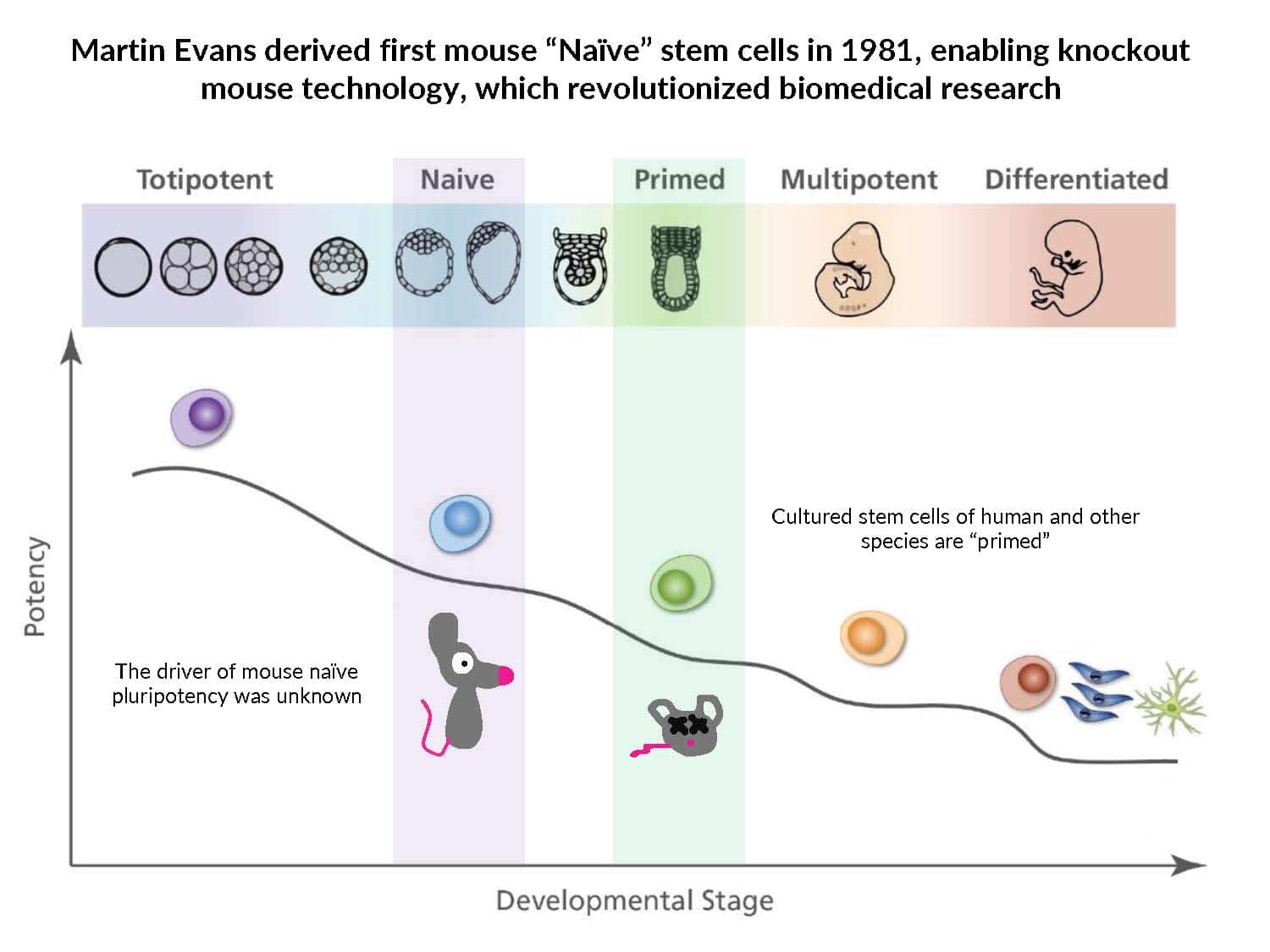
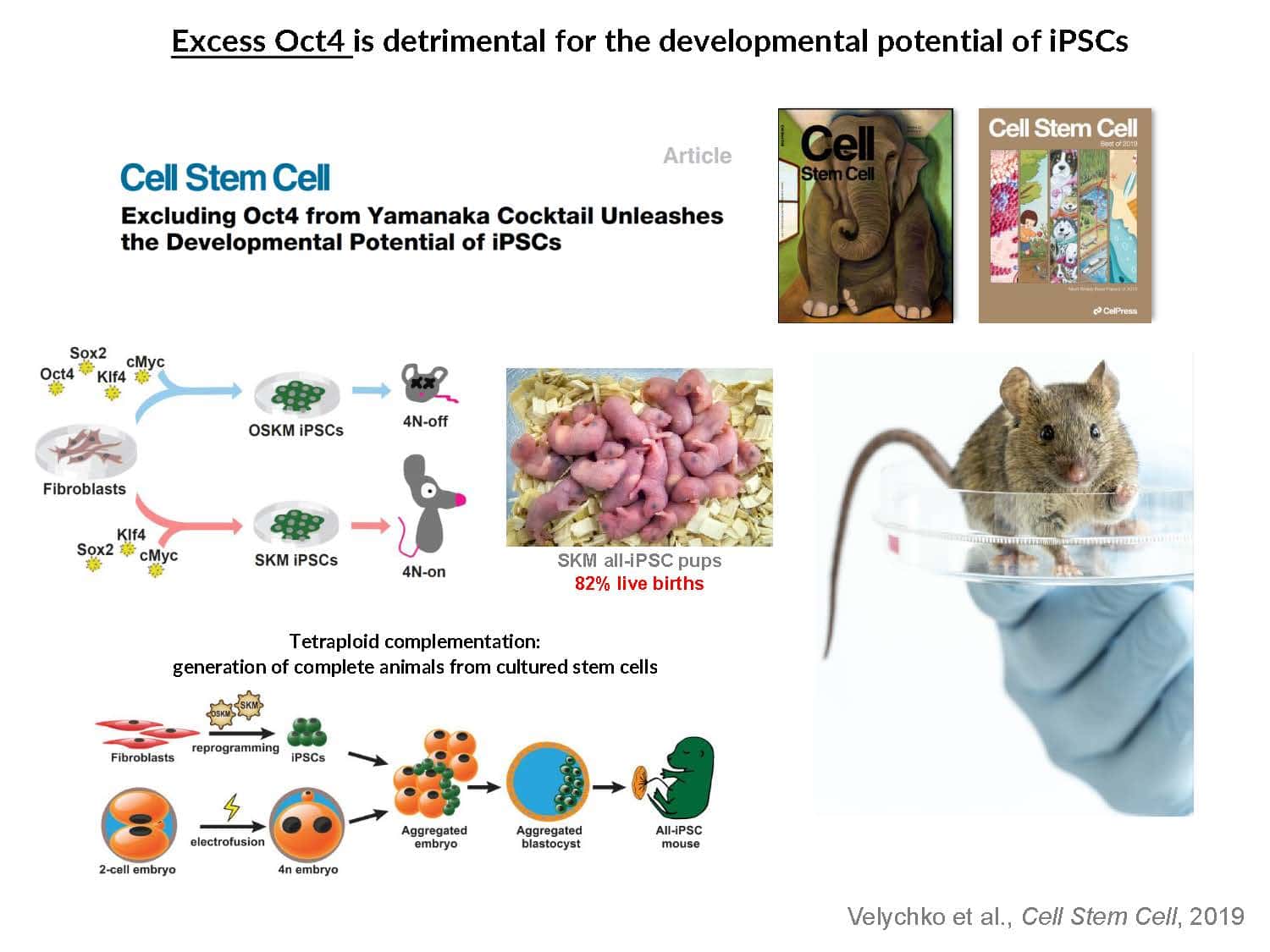
Our understanding of pluripotency remains limited: iPSC generation has only been established for a few species, pluripotent stem cell lines exhibit inconsistent developmental potential, and germline transmission has only been demonstrated for mice and rats.
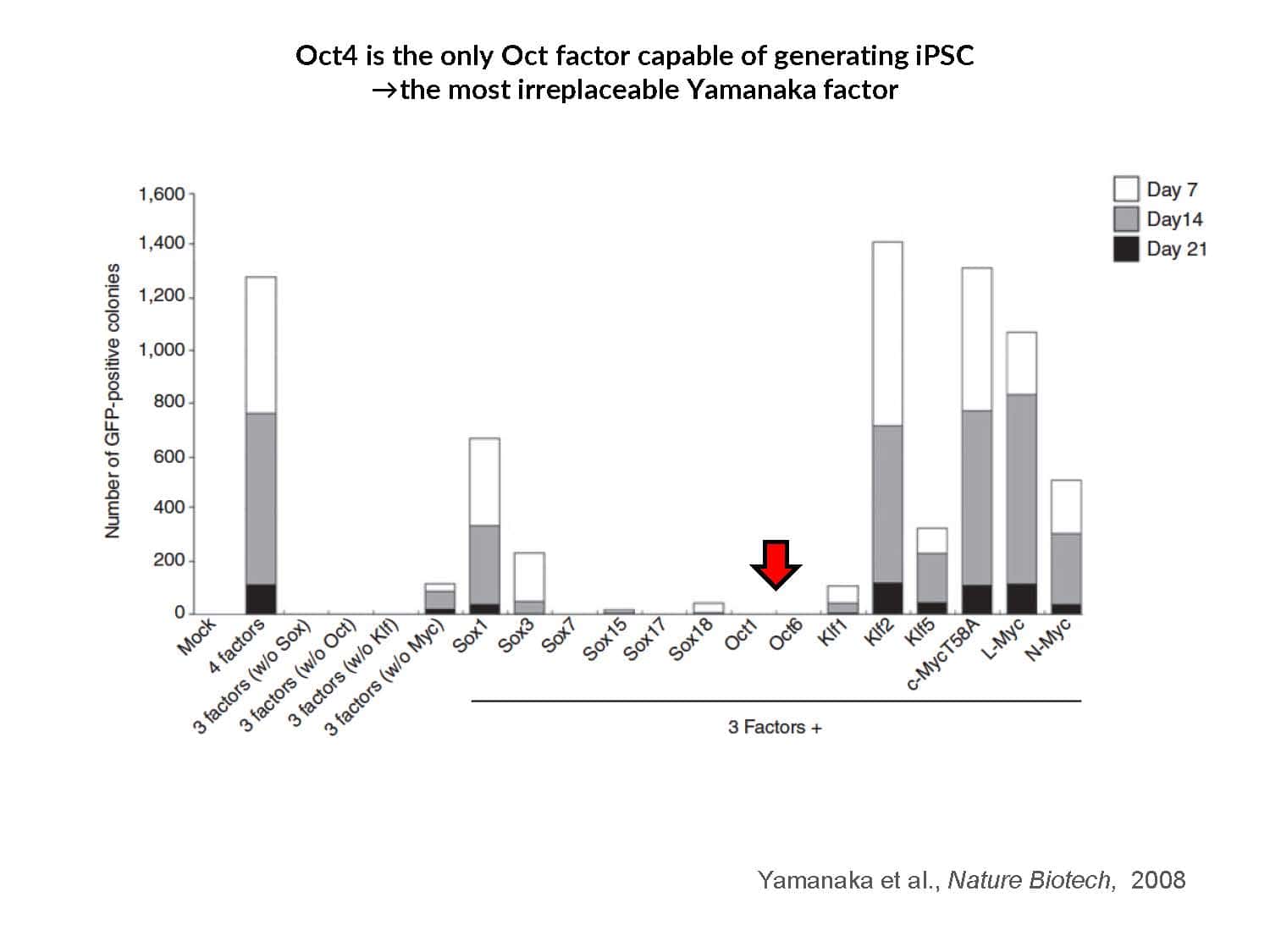
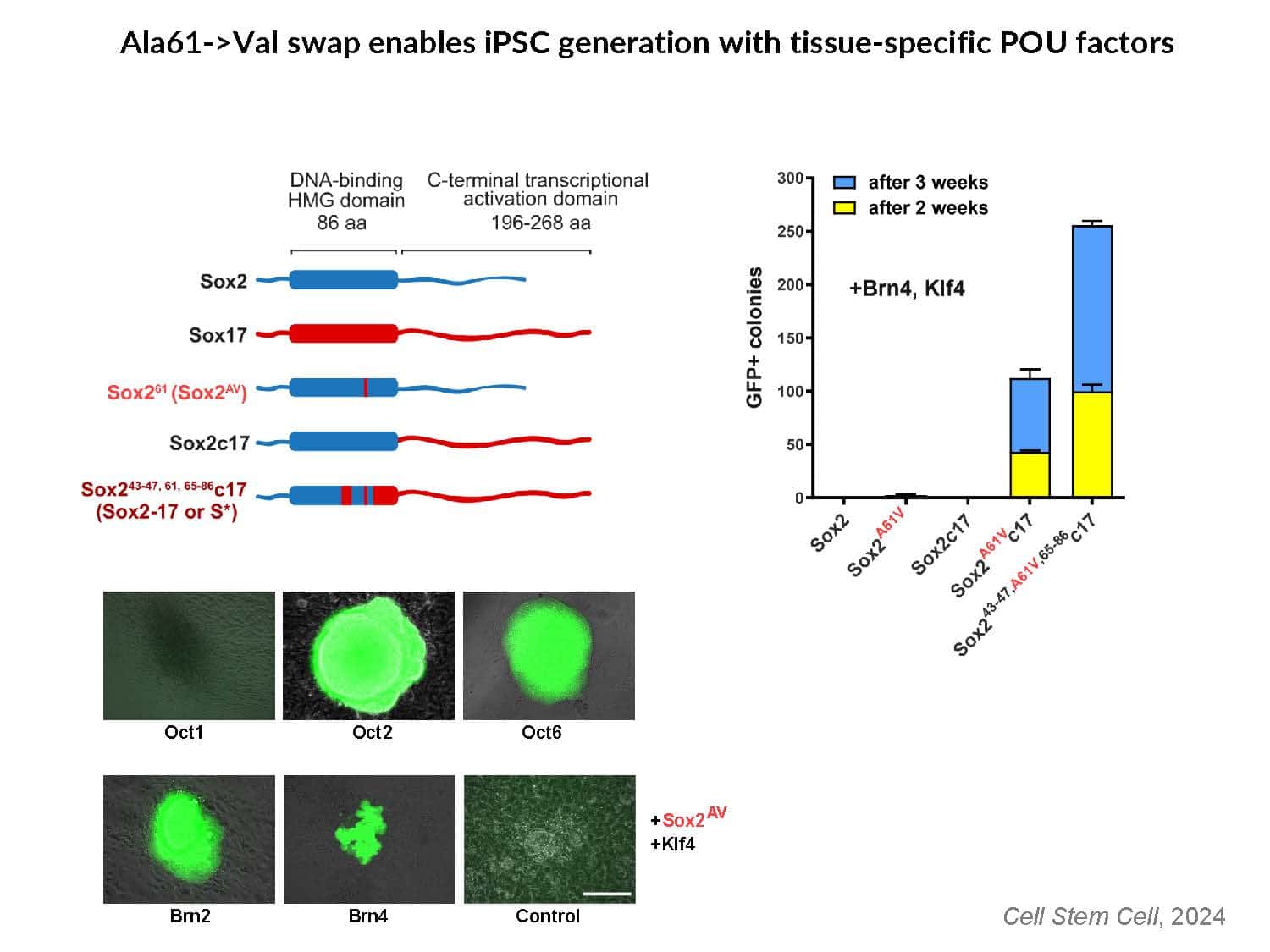
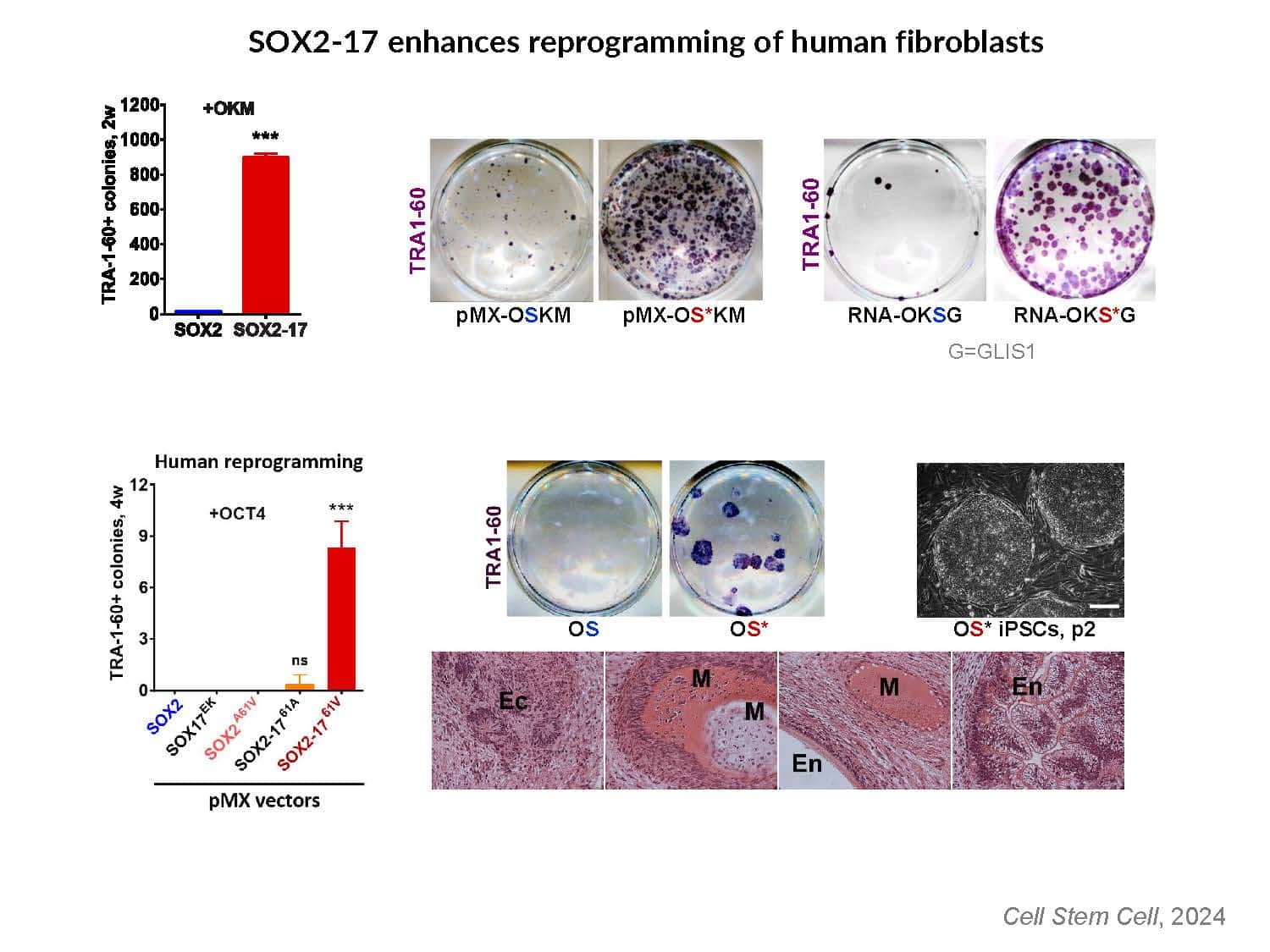
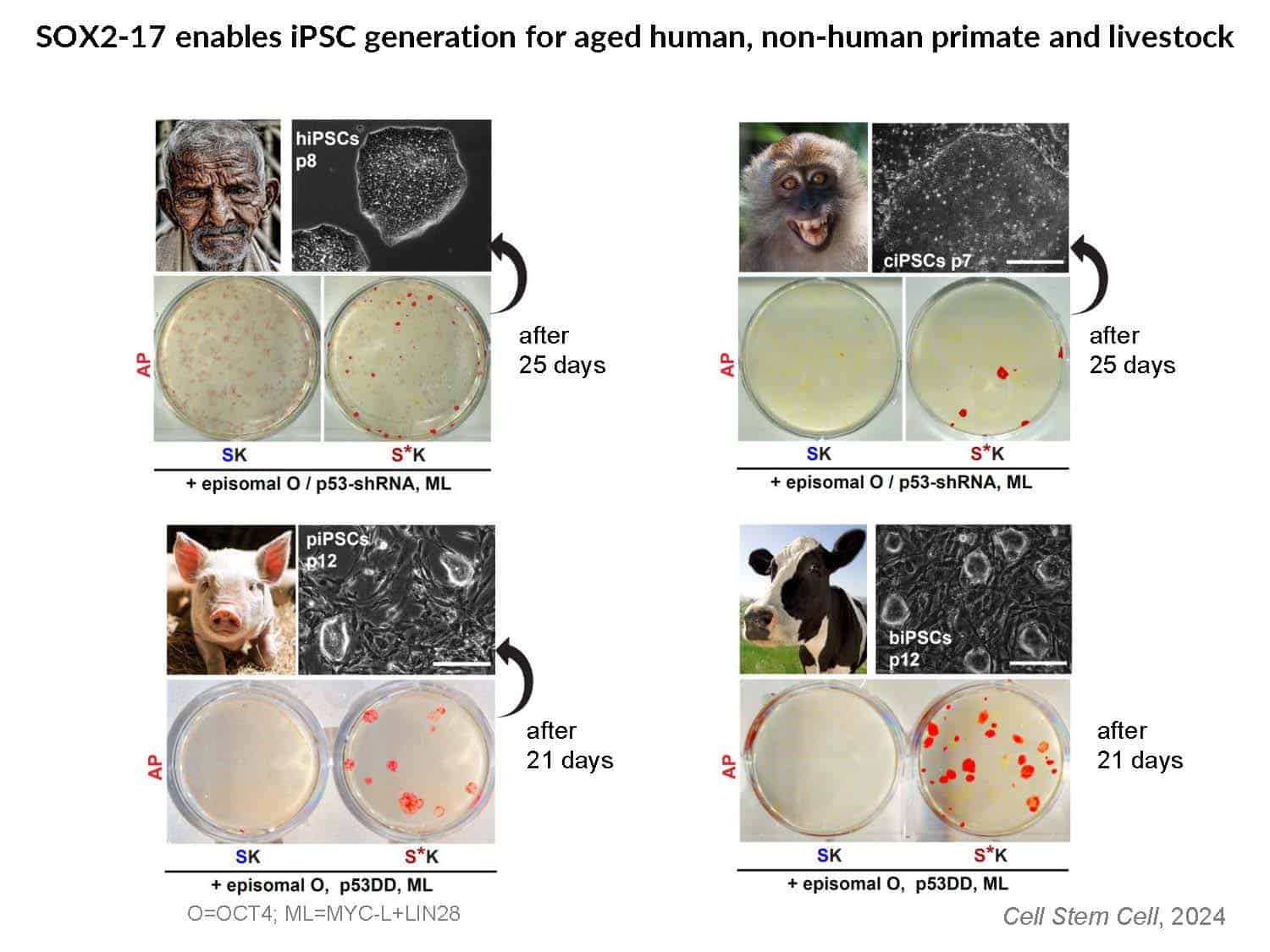
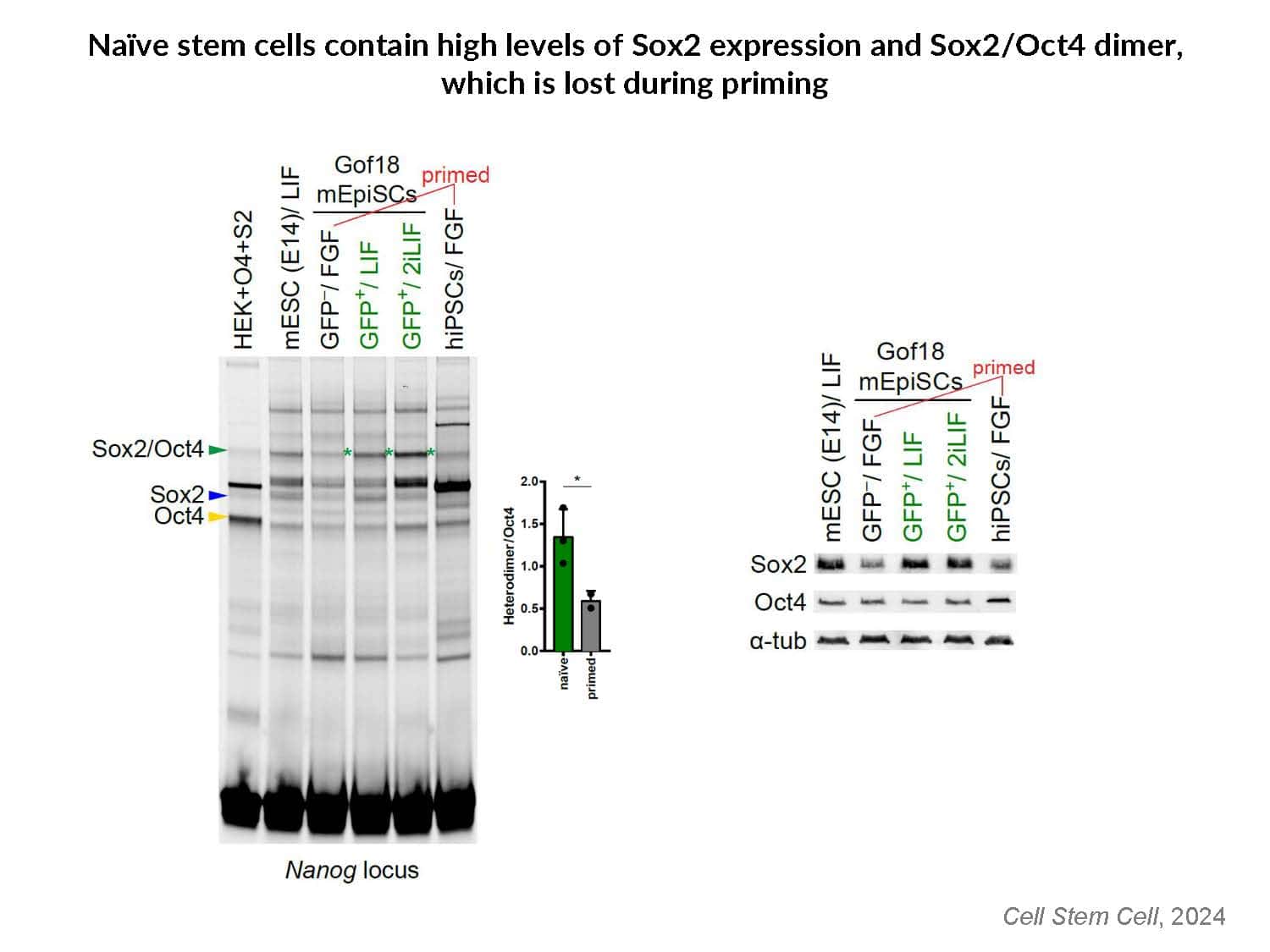
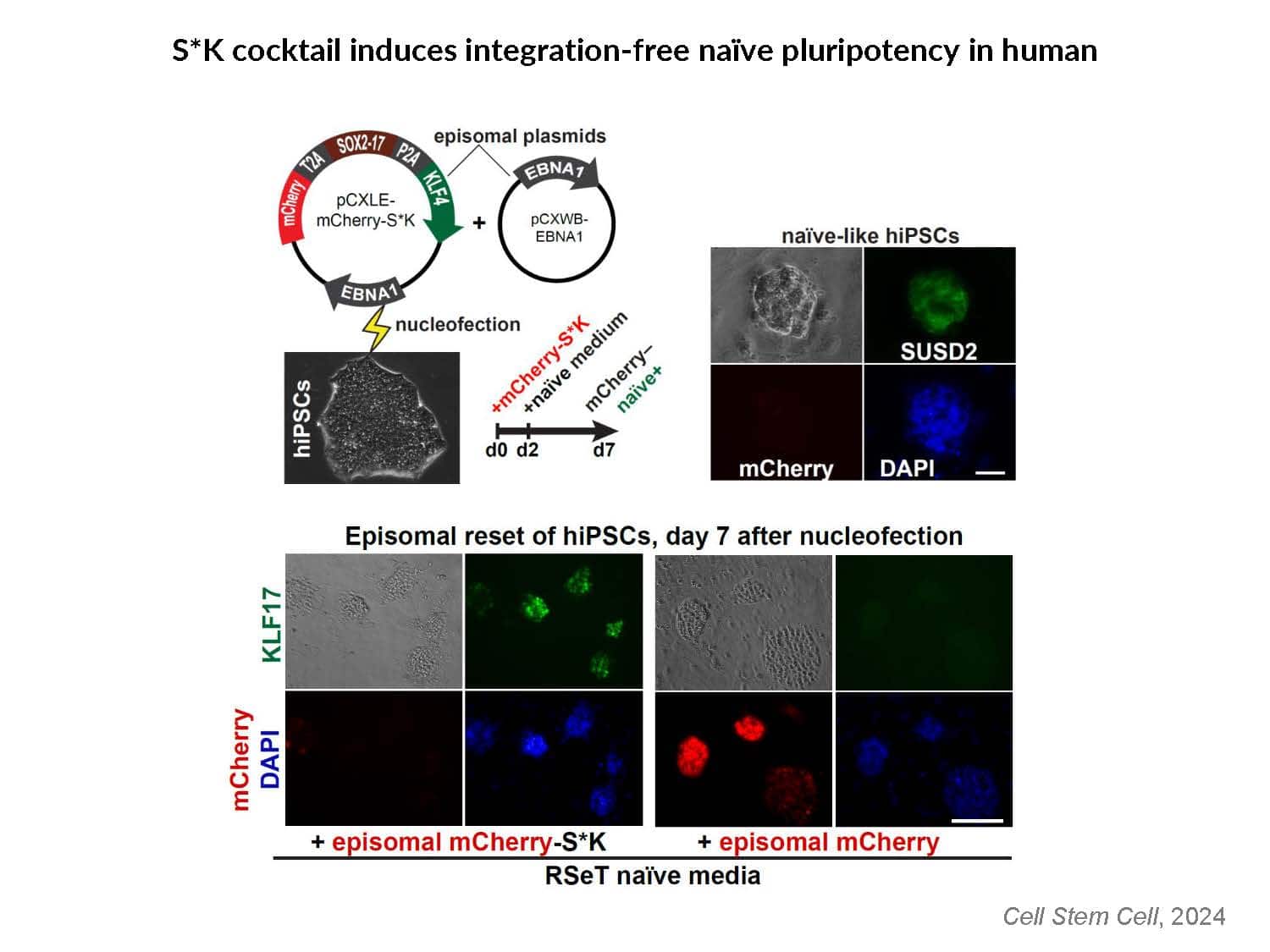
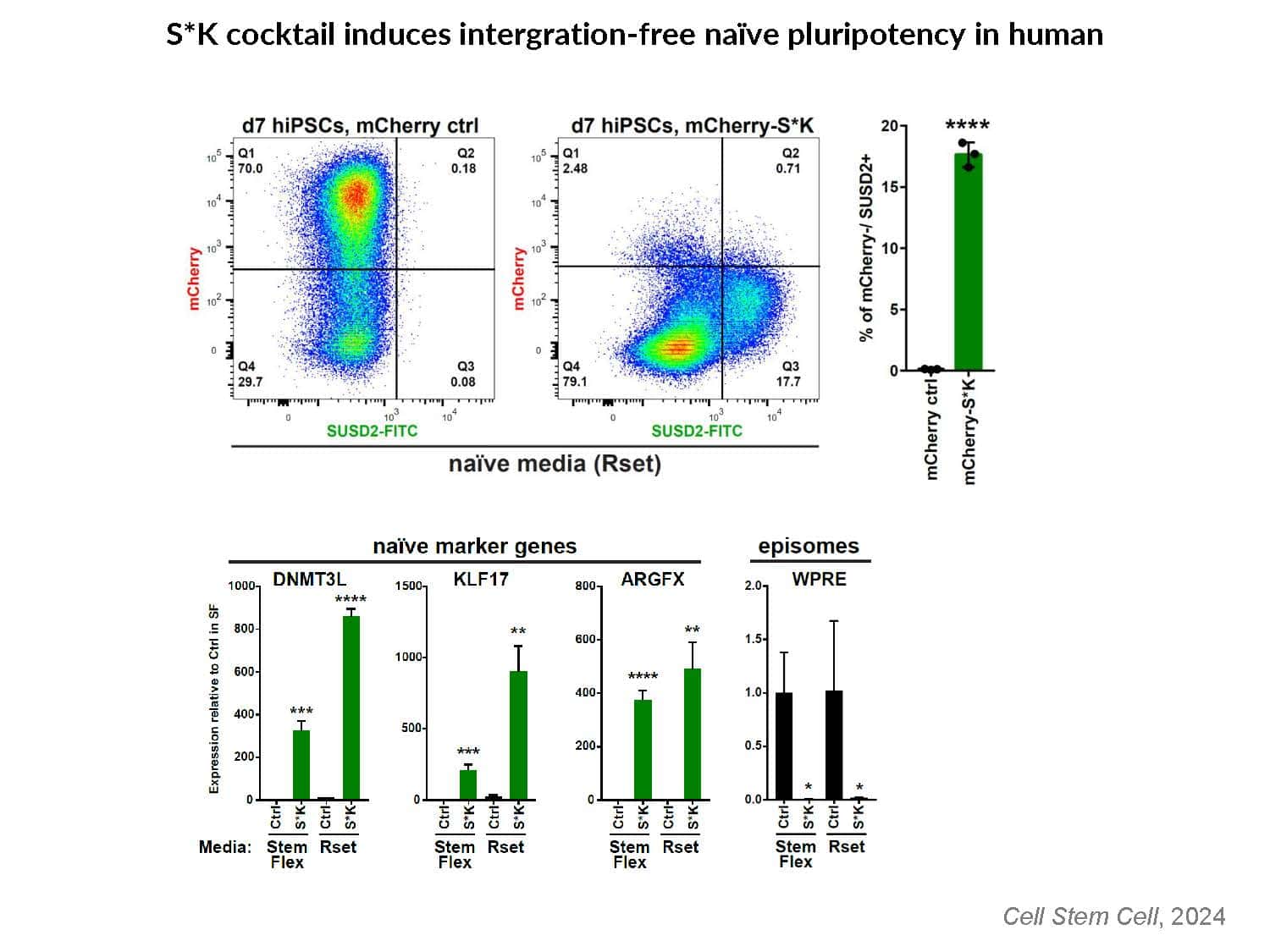
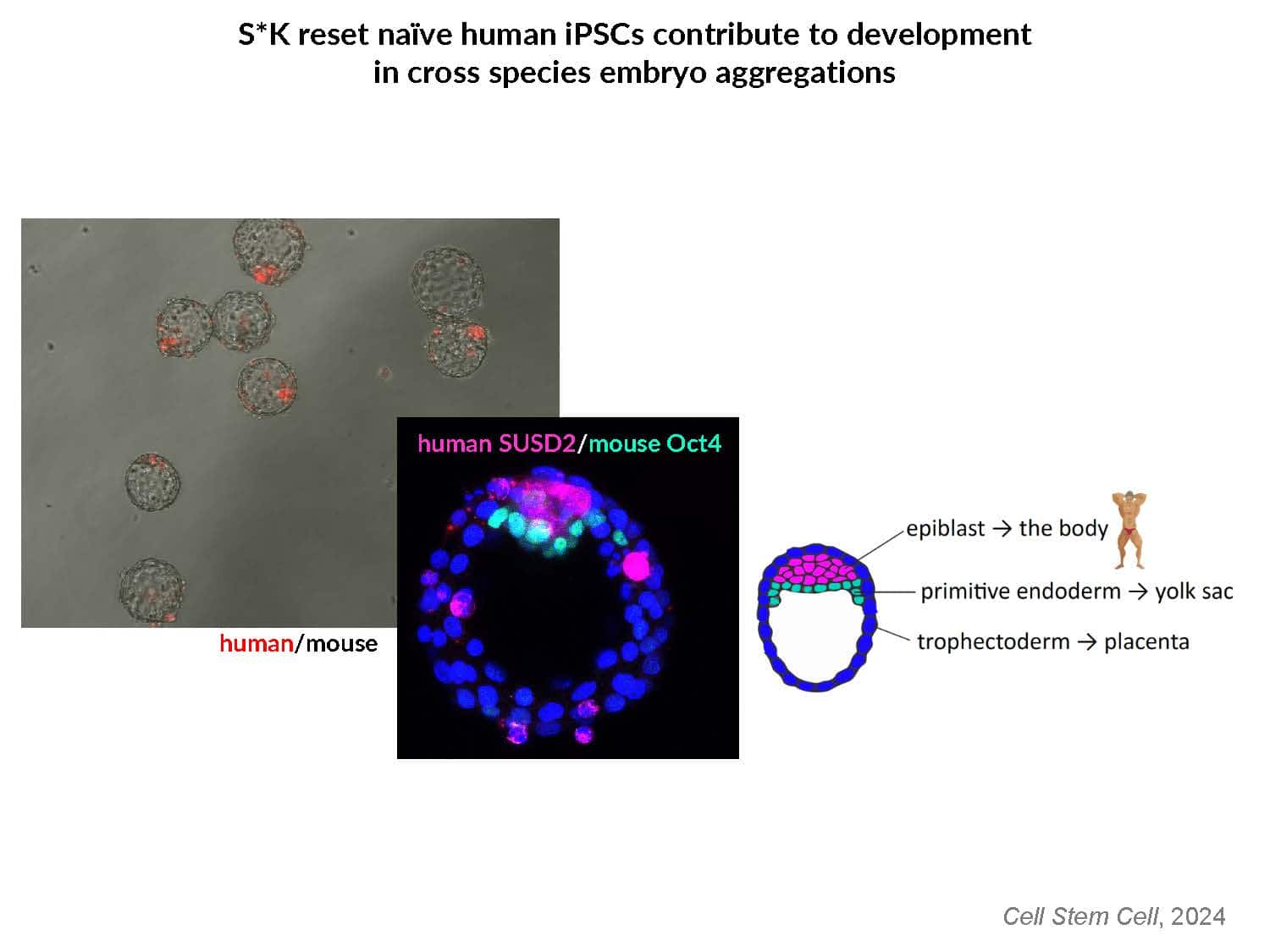
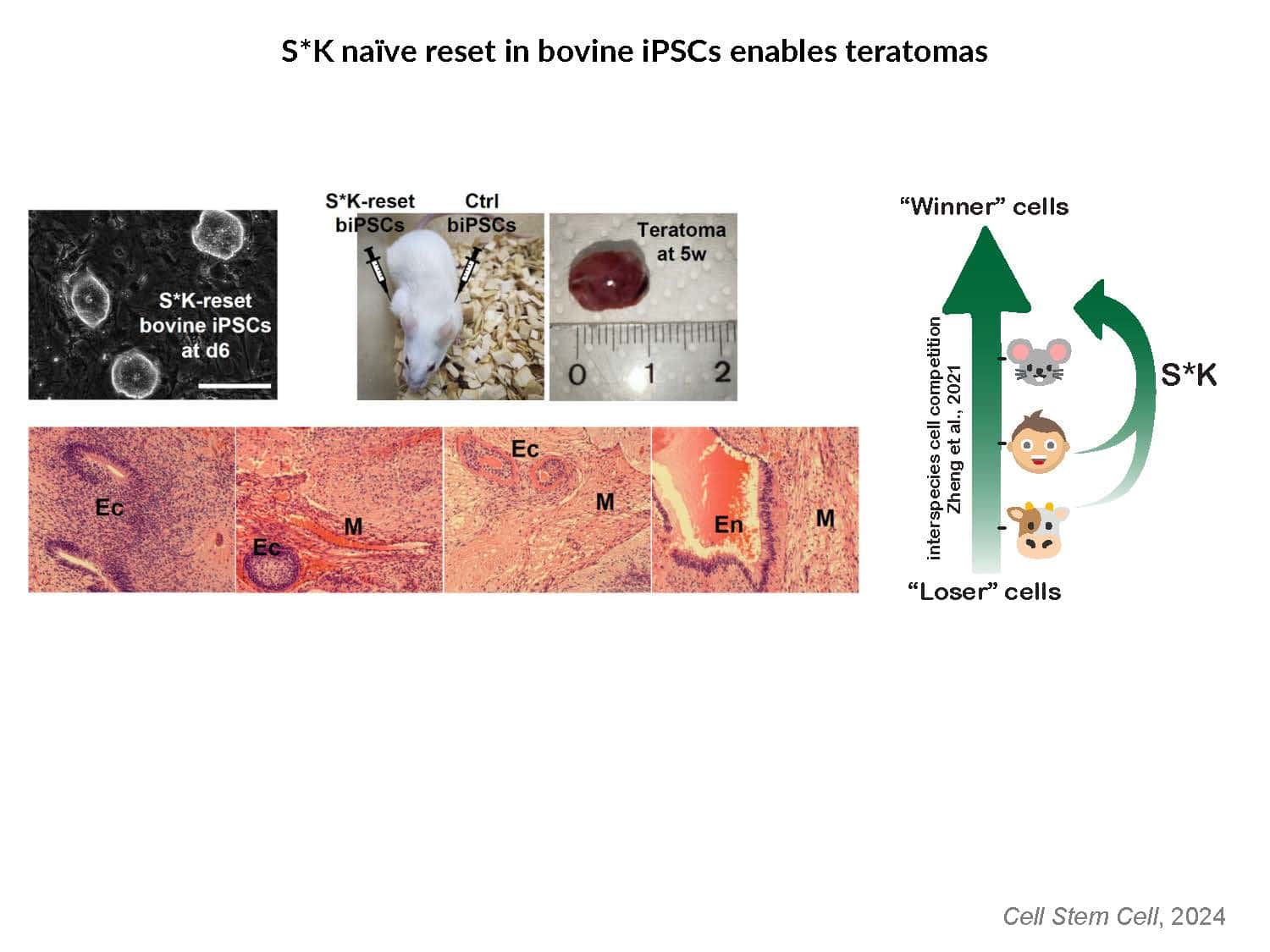
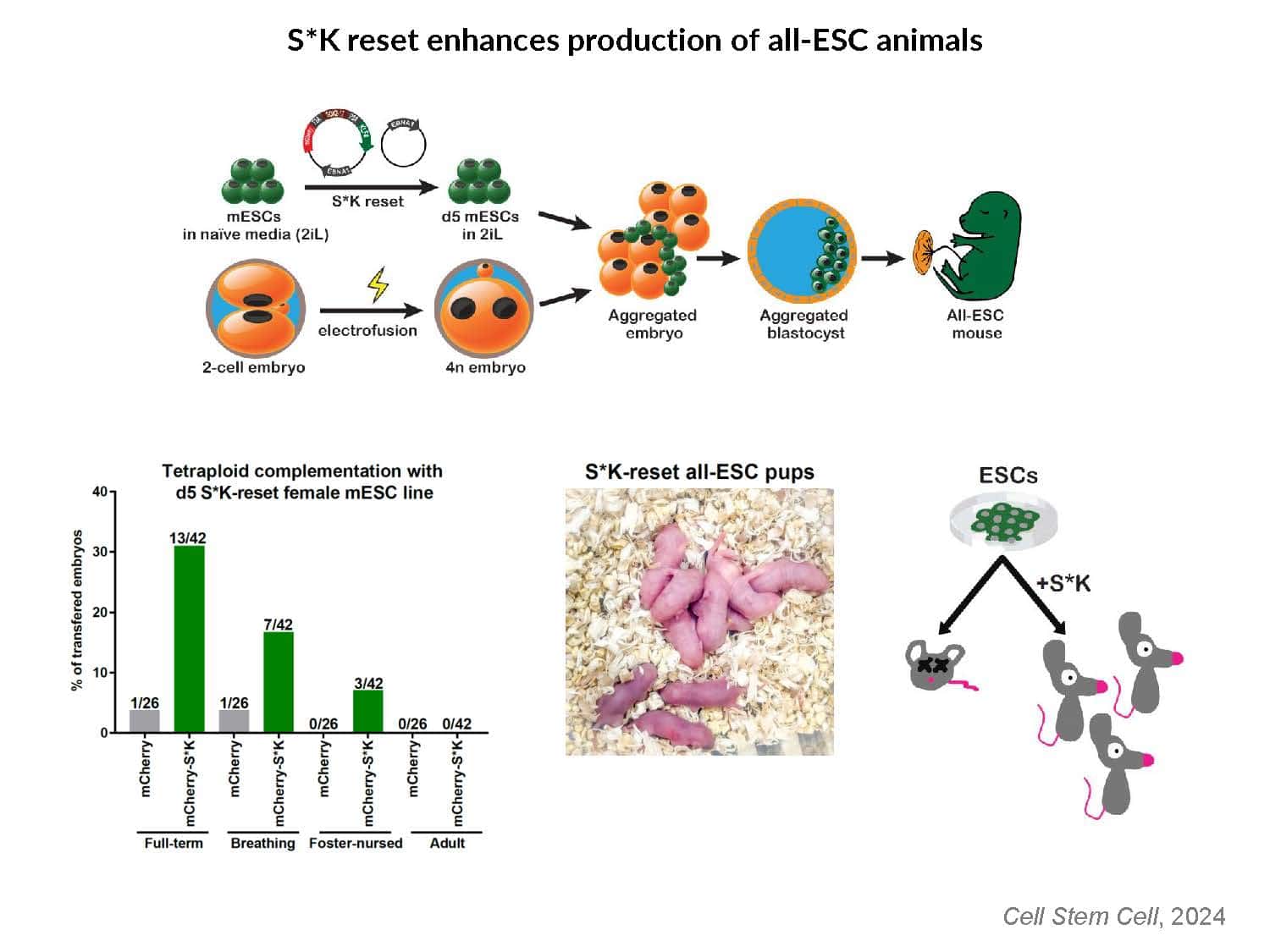
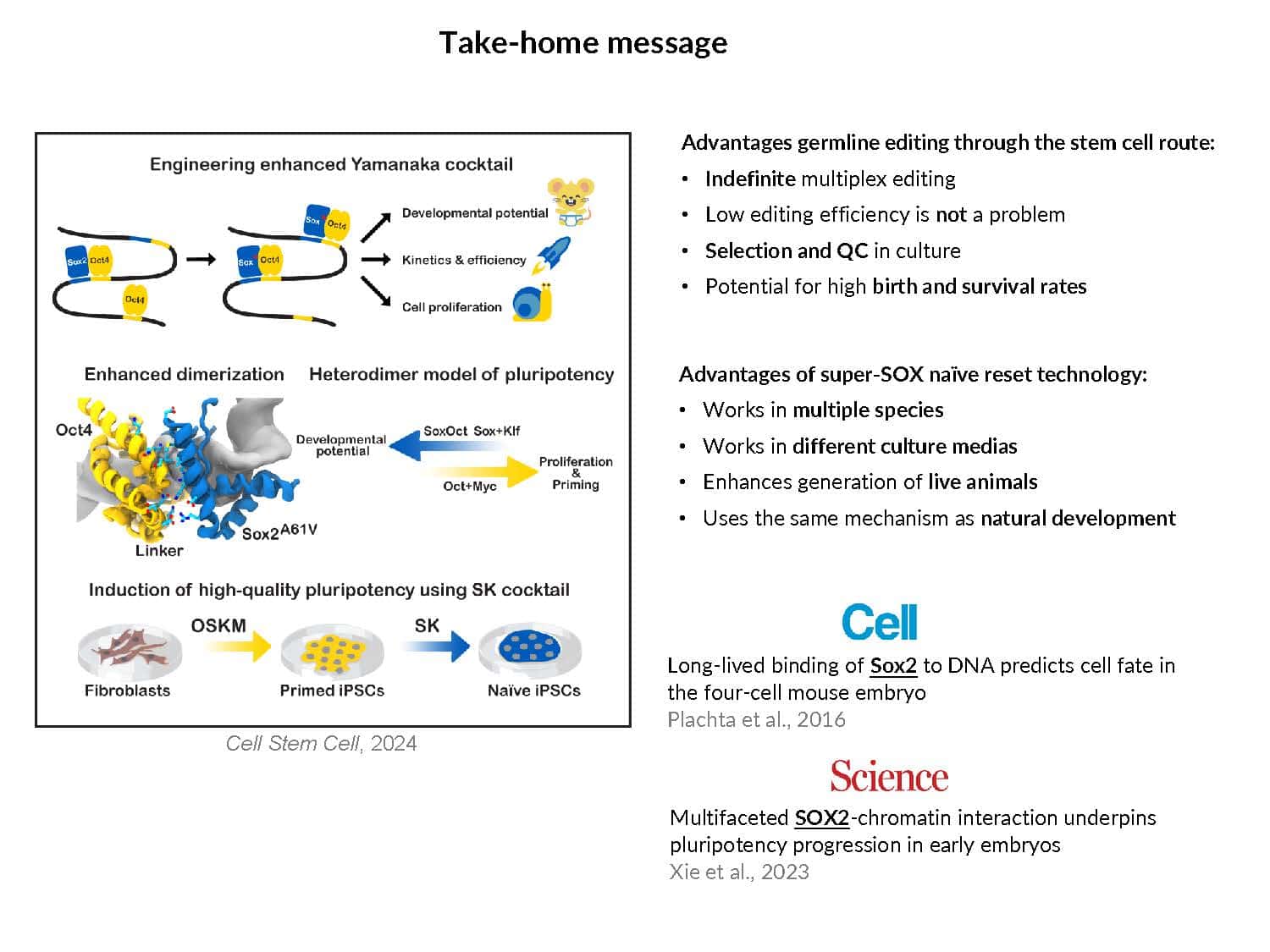
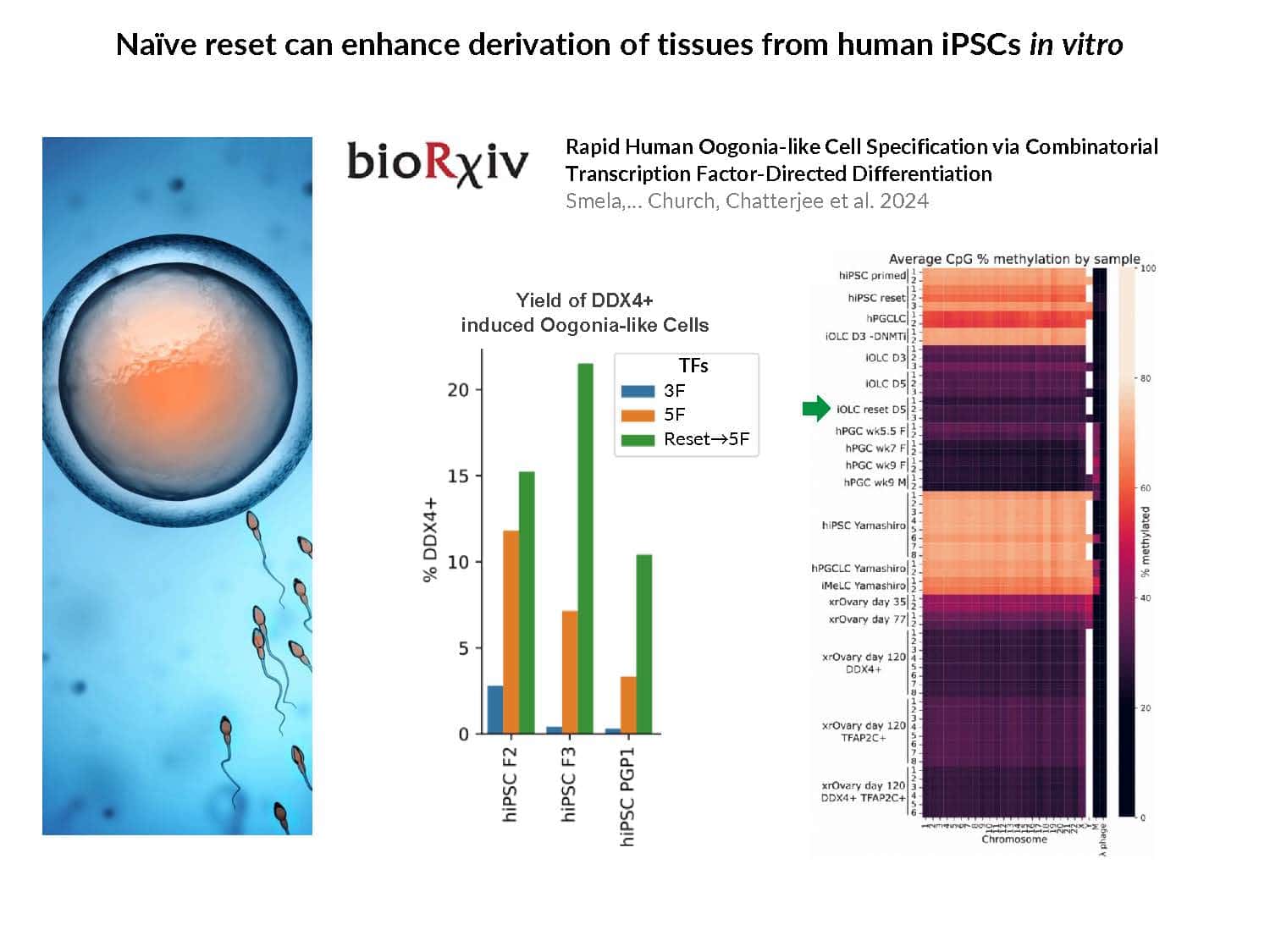
By swapping structural elements between Sox2 and Sox17, we created a chimeric super-reprogramming factor, Sox2-17, which enhanced iPSC generation in five tested species: mice, humans, cynomolgus monkeys, cows, and pigs. The most crucial gain-of-function came from a single residue swap, alanine to valine at position 61 of the Sox2-HMG domain facing Oct4. Our computer simulations, biochemistry, and ChIP-seq experiments demonstrated that Sox2-A61V stabilizes Sox2/Oct4 dimerization on SoxOct DNA motif that controls the pluripotency fate. The point mutant markedly boosted the developmental potential of OSKM iPSCs, as evidenced by their much-improved capacity to support the generation of healthy all-iPSC mice in tetraploid complementation experiments. Sox2/Oct4 dimer emerged as the core driver of naïve pluripotency both during development and in vitro, with the dimer levels diminishing upon priming. Transient overexpression of episomal SK (super-SOX+KLF4 cocktail) restores endogenous Sox2/Oct4 dimerization, enhancing the developmental potential of existing pluripotent stem cell lines across different species. Our research provides a powerful tool for advancing iPSC technology, presents a universal approach for the naive reset across species, and addresses the long-standing question of what dictates the developmental potential or “quality” of pluripotent stem cells. By enabling the generation of high-quality naive pluripotent cells, our findings could enhance the production of tissues and organs and pave the way for precision mammalian germline engineering beyond the mouse model.