As current day nanotechnology makes incremental advances in technologies that advance the goal of atomic precision in control of the structure of matter, such as DNA nanotechnology, such advances sometimes also provide opportunities to apply primitive nanomachines to current needs. A hat tip to KurzweilAI for reporting such an advance announced by the newsroom of the Université de Montréal “Detecting HIV diagnostic antibodies with DNA nanomachines“:
New research may revolutionize the slow, cumbersome and expensive process of detecting the antibodies that can help with the diagnosis of infectious and auto-immune diseases such as rheumatoid arthritis and HIV. An international team of researchers have designed and synthesized a nanometer-scale DNA “machine” whose customized modifications enable it to recognize a specific target antibody. Their new approach, which they described this month in Angewandte Chemie [abstract], promises to support the development of rapid, low-cost antibody detection at the point-of-care, eliminating the treatment initiation delays and increasing healthcare costs associated with current techniques.
The binding of the antibody to the DNA machine causes a structural change (or switch), which generates a light signal. The sensor does not need to be chemically activated and is rapid – acting within five minutes – enabling the targeted antibodies to be easily detected, even in complex clinical samples such as blood serum.
“One of the advantages of our approach is that it is highly versatile,” said Prof. Francesco Ricci, of the University of Rome, Tor Vergata, senior co-author of the study. “This DNA nanomachine can be in fact custom-modified so that it can detect a huge range of antibodies, this makes our platform adaptable for many different diseases”.
“Our modular platform provides significant advantages over existing methods for the detection of antibodies,” added Prof. Vallée-Bélisle of the University of Montreal, the other senior co-author of the paper. “It is rapid, does not require reagent chemicals, and may prove to be useful in a range of different applications such as point-of-care diagnostics and bioimaging”.
“Another nice feature of our this platform is its low-cost,” said Prof. Kevin Plaxco of the University of California, Santa Barbara. “The materials needed for one assay cost about 15 cents, making our approach very competitive in comparison with other quantitative approaches.”
“We are excited by these preliminary results, but we are looking forward to improve our sensing platform even more” said Simona Ranallo, a PhD student in the group of Prof. Ricci at the University of Rome and first-author of the paper. “For example, we could adapt our platform so that the signal of the nanoswitch may be read using a mobile phone. This will make our approach really available to anyone! We are working on this idea and we would like to start involving diagnostic companies.”
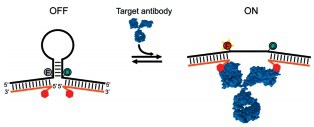
The concept for this simple nanomachine is elegant enough: a light-emitting molecule is held by a weak DNA stem close to a molecule that quenches its signal. Attached near each end of the stem are two recognition elements—peptides, oligonucleotides, or other small molecules— that recognize widely separated regions on the target molecule, or on two separate target molecules. When these recognition elements bind to their targets, the weak DNA stem is broken appart, separating the quencher and fluorophore, and thus allowing the light signal to be emitted.
The success of the application will probably depend upon the sensitivity and precision of the two target recognition elements. Initial results reported in the publication indicate good sensitivity and no cross-reaction with seven target proteins representing several different types of proteins. If this application proves faster, more accurate, more convenient, and less expensive than alternative methods, it may pave the way for a wider variety of ever more sophisticated atomically precise nanomachines for diagnosis and therapy, leading eventually to complex medical nanorobots incorporating sensing, diagnosis, mobility, and therapeutic functions.
—James Lewis, PhD