One of the most promising current applications of nanotechnology to medicine is the use of nanoparticles to specifically target drug therapy to cancer cells. A variety of different types of nanoparticles using different drug delivery strategies are being investigated, including one type using biopolymers that we described here last week. Another report shows that a very different type of nanoparticle, composed of gold, works by delivering a drug directly to the nucleus of cancer cells. A hat tip to ScienceDaily for reprinting this news release from Northwestern University written by Megan Fellman “Tiny hitchhikers attack cancer cells: Gold nanostars first to deliver drug directly to cancer cell nucleus“:
Nanotechnology offers powerful new possibilities for targeted cancer therapies, but the design challenges are many. Northwestern University scientists now are the first to develop a simple but specialized nanoparticle that can deliver a drug directly to a cancer cell’s nucleus — an important feature for effective treatment.
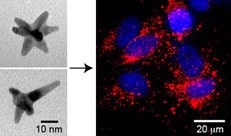
They also are the first to directly image at nanoscale dimensions how nanoparticles interact with a cancer cell’s nucleus.
“Our drug-loaded gold nanostars are tiny hitchhikers,” said Teri W. Odom, who led the study of human cervical and ovarian cancer cells. “They are attracted to a protein on the cancer cell’s surface that conveniently shuttles the nanostars to the cell’s nucleus. Then, on the nucleus’ doorstep, the nanostars release the drug, which continues into the nucleus to do its work.” …
Using electron microscopy, Odom and her team found their drug-loaded nanoparticles dramatically change the shape of the cancer cell nucleus. What begins as a nice, smooth ellipsoid becomes an uneven shape with deep folds. They also discovered that this change in shape after drug release was connected to cells dying and the cell population becoming less viable — both positive outcomes when dealing with cancer cells.
The results are published in the journal ACS Nano [abstract].
Since this initial research, the researchers have gone on to study effects of the drug-loaded gold nanostars on 12 other human cancer cell lines. The effect was much the same. “All cancer cells seem to respond similarly,” Odom said. “This suggests that the shuttling capabilities of the nucleolin protein for functionalized nanoparticles could be a general strategy for nuclear-targeted drug delivery.”
The nanoparticle is simple and cleverly designed. It is made of gold and shaped much like a star, with five to 10 points. (A nanostar is approximately 25 nanometers wide.) The large surface area allows the researchers to load a high concentration of drug molecules onto the nanostar. Less drug would be needed than current therapeutic approaches using free molecules because the drug is stabilized on the surface of the nanoparticle.
The drug used in the study is a single-stranded DNA aptamer called AS1411. Approximately 1,000 of these strands are attached to each nanostar’s surface.
The DNA aptamer serves two functions: it is attracted to and binds to nucleolin, a protein overexpressed in cancer cells and found on the cell surface (as well as within the cell). And when released from the nanostar, the DNA aptamer also acts as the drug itself.
Bound to the nucleolin, the drug-loaded gold nanostars take advantage of the protein’s role as a shuttle within the cell and hitchhike their way to the cell nucleus. The researchers then direct ultrafast pulses of light — similar to that used in LASIK surgery — at the cells. The pulsed light cleaves the bond attachments between the gold surface and the thiolated DNA aptamers, which then can enter the nucleus.
In addition to allowing a large amount of drug to be loaded, the nanostar’s shape also helps concentrate the light at the points, facilitating drug release in those areas. Drug release from nanoparticles is a difficult problem, Odom said, but with the gold nanostars the release occurs easily.
That the gold nanostar can deliver the drug without needing to pass through the nuclear membrane means the nanoparticle is not required to be a certain size, offering design flexibility. Also, the nanostars are made using a biocompatible synthesis, which is unusual for nanoparticles.
Odom envisions the drug-delivery method, once optimized, could be particularly useful in cases where tumors are fairly close to the skin’s surface, such as skin and some breast cancers. (The light source would be external to the body.) Surgeons removing cancerous tumors also might find the gold nanostars useful for eradicating any stray cancer cells in surrounding tissue.
A particular advantage of these nanostars is that the plasmonic electrons produced on the surface of the nanostars by the laser solves the problem of how to efficiently discharge the drug target from the nanoparticle vehicle.
—James Lewis, PhD