Summary
In this session, Jean Hebert from the Albert Einstein School of Medicine introduced the idea of brain tissue replacement as a necessary strategy for the long-term defeat of aging. He went through the history of the field and research so far and presented a case for pursuing this approach further based on the available positive data. He also talked about the roadmap, including a pioneering experiment, and next steps for which they are now looking for funders.
Presenters

Jean Hebert, Albert Einstein School of Medicine
Jean Hebert is Professor in the Dominick P. Purpura Department of Neuroscience, and Professor in the Department of Genetics. His research is focused on devising methods of cell replacement for the adult neocortex when its cells are lost due to damage or age-related degeneration…
Presentation: Brain Cell Replacement to Beat Aging
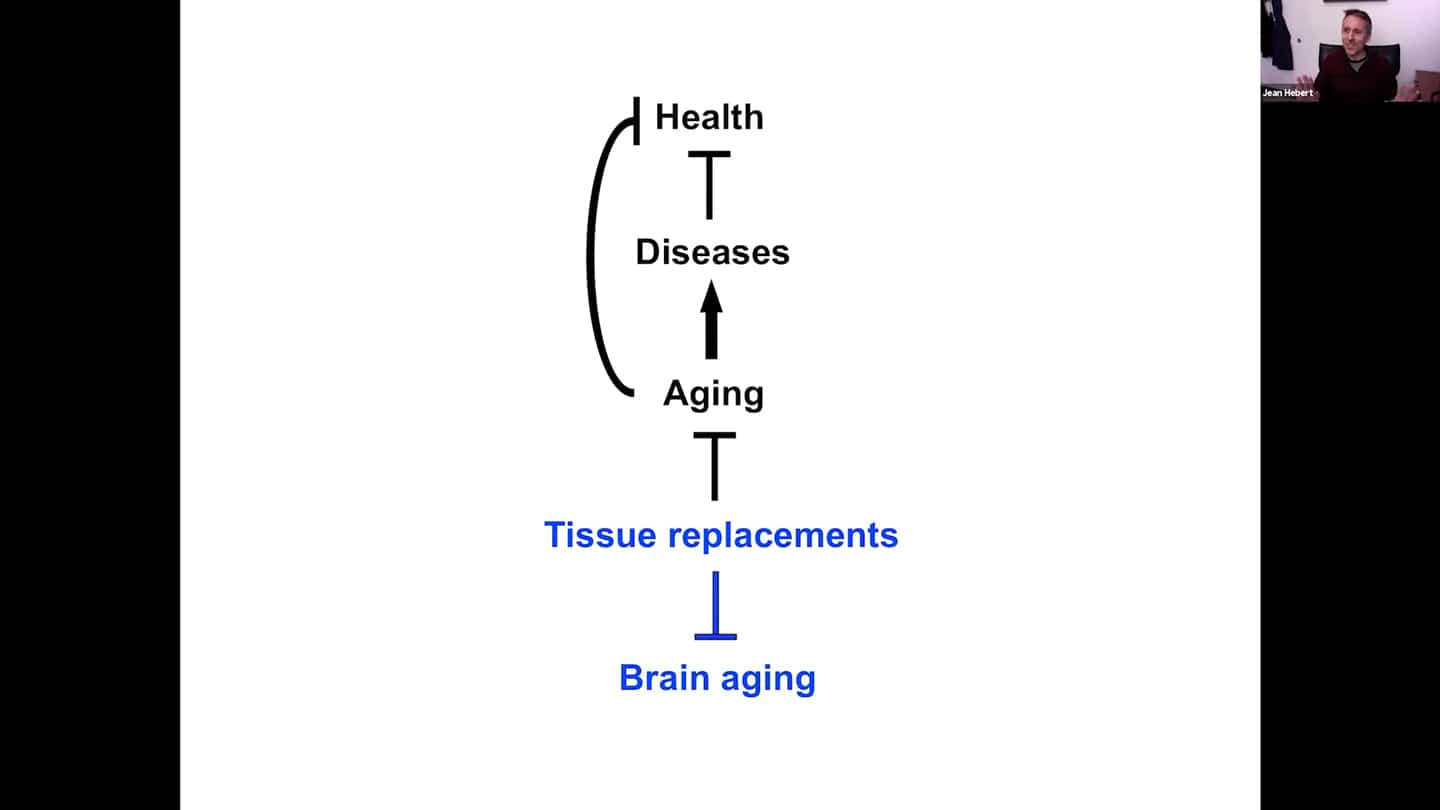
- Tissue replacement (cells, tissues, organs,…) is not a new idea, it’s one of possible approaches to defeat aging. And if replacements are ever going to be useful, we need to figure out how to apply it to the brain. And it’s the time to start doing that, because it seems to be doable.
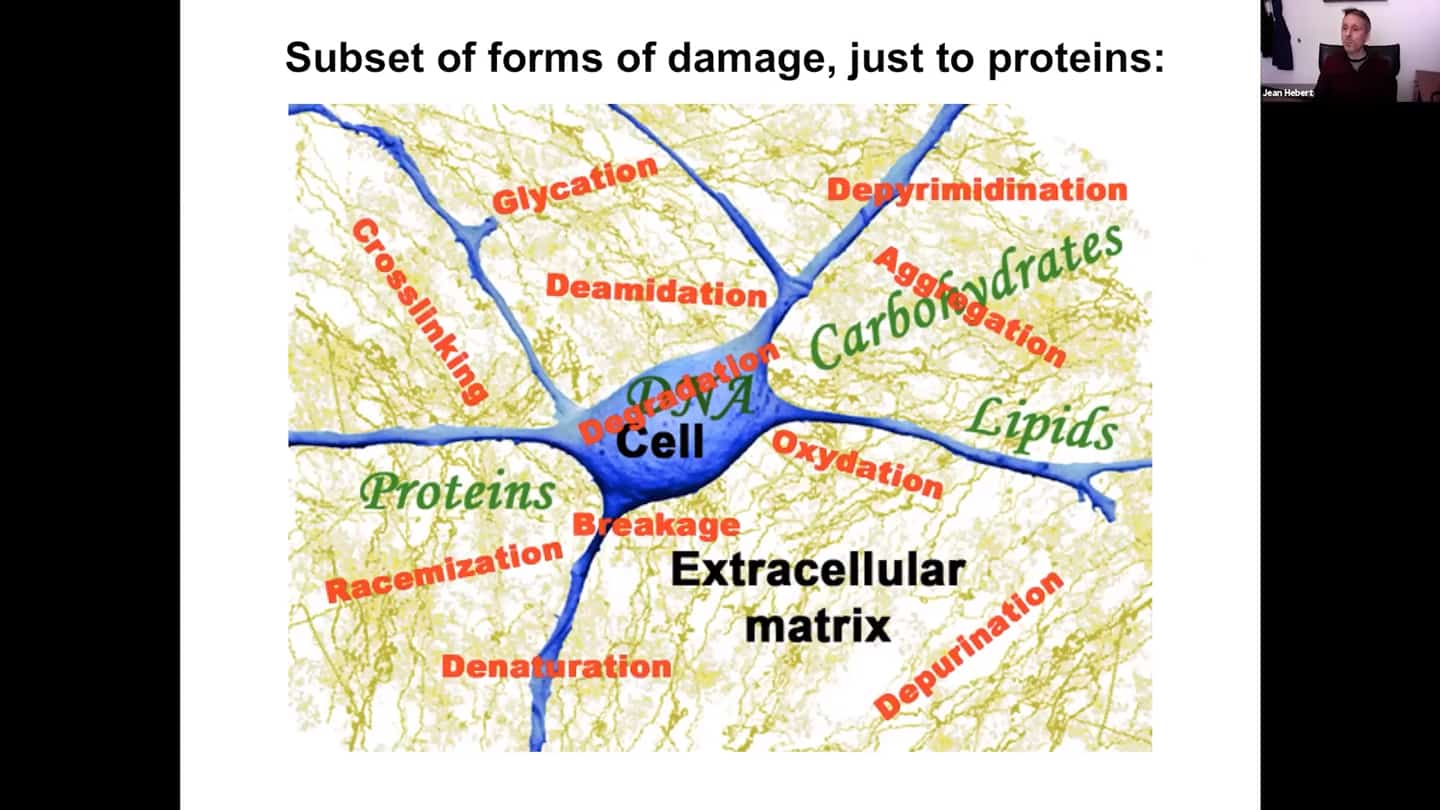
- Aging is the accumulation of macromolecular damage over time. This damage is very complex, there are many different forms of damage. Damage affects many different intracellular pathways, and there’s a lot more basic research needed to be done to understand all the different pathways and ways how they are affected, and how we could reset them using combinations of drugs or pharmacological agents.
- We need to acknowledge the limitations of that approach to tackle aging. Finding combinations of drugs that will reset that many pathways within cells – for delicately balanced life sustaining processes – without side effects that would outweigh the benefits is a hard thing to imagine. Even if we did manage to do that, we are not addressing the cause itself, which is the accumulation of damage itself. We might live a bit longer and healthier, but we won’t defeat aging.
- To defeat aging, we need a replacement approach.
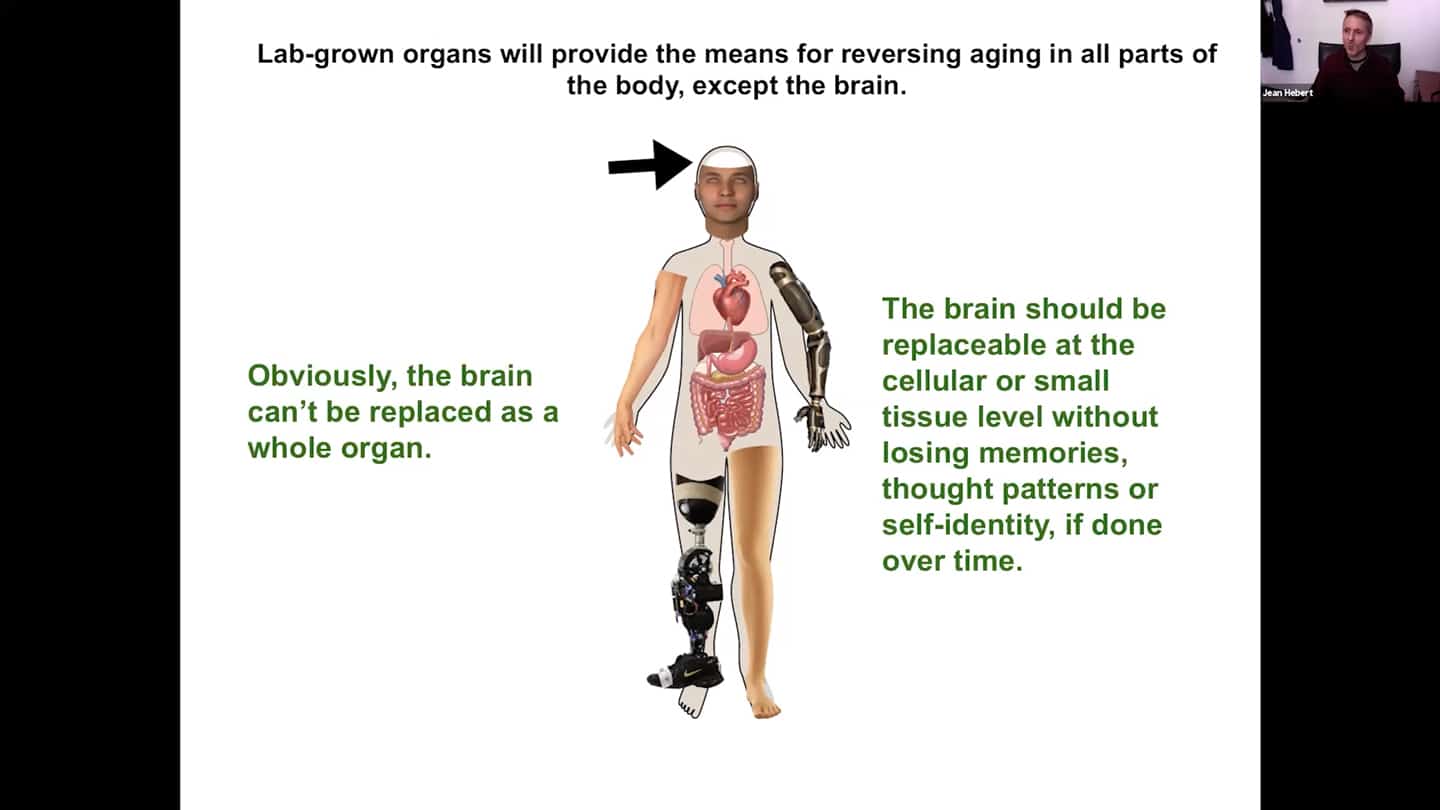
- Replacements per se, in terms of implementation, are not that problematic. Every part of the body was surgically replaced over the last few decades to treat disease and injury, so technically it’s not that big of a problem. Problem is with the replacements themselves, so there is a need for lab grown organs and improved prosthetics. Progress is painfully slow, investments are rather limited. But eventually we will be able to replace all parts of the body. Except the brain of course. And you of course don’t want to live in a young body with a senile brain. It cannot be replaced as a whole organ, but the brain should be replaceable progressively via brain cell replacements without losing our identity.
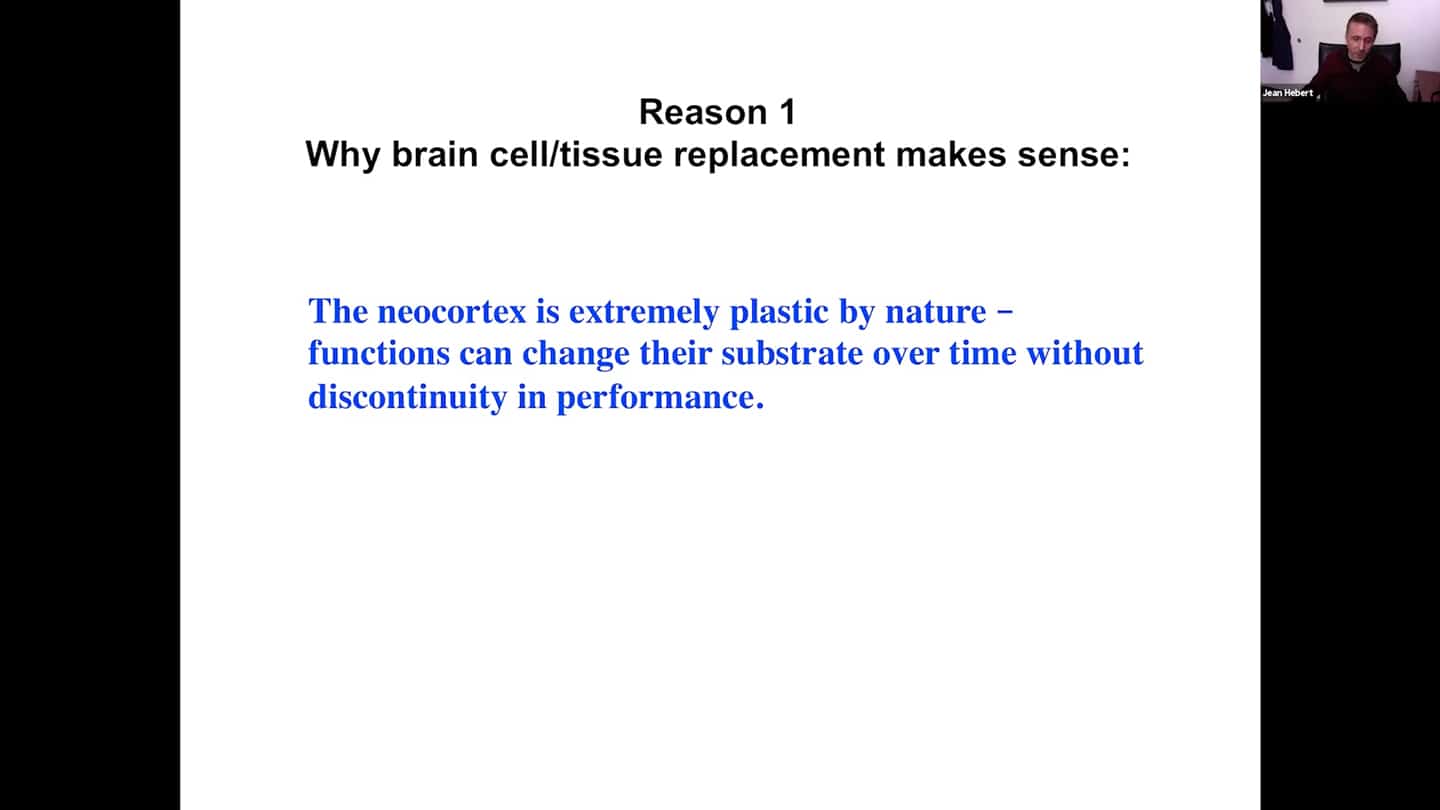
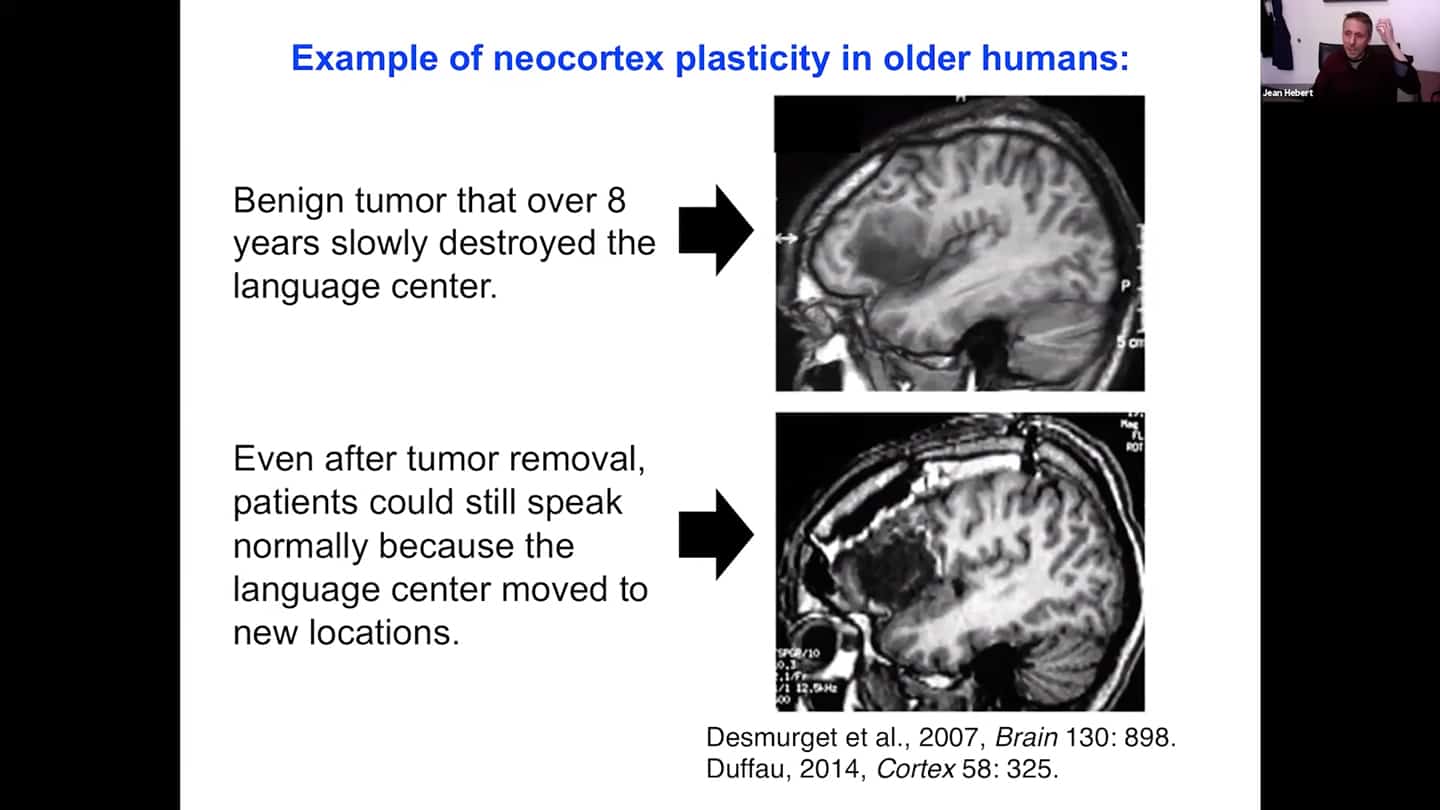
- The human brain is mainly neocortex. That’s where our thought patterns and self-identity are stored. The neocortex is extremely plastic by nature, functions can change their substrate over time.
- Cases where even older patients 50 or 60 years old had their benign tumor removed from the brain, and the tumor was where the language centre was. We observed that the transfer of function must’ve happened progressively because they didn’t lose the ability to speak. Therefore it should be possible to do this progressively with an intervention that provides young naive tissue over time as well.
- The other reason why tissue replacements make sense is the evidence from studies that were putting in immature precursor cells for the neocortex and showing that these immature precursors differentiate into neurons that make the appropriate connections to distant parts of the brain in model animals.
- General process looks like this:
- Aging brain loses a lot of volume, which creates space.
- In that space we could implant new grafted functional tissue, assuming we can make it. Lots of labs are already grafting tissue, it’s just not that functional yet, but it’s a work in progress.
- We could silence the old tissue and the area of neocortex we want to get rid of, that is nearby the new grafted one. That should be possible pharmacologically.
- Then we could remove that old tissue once it’s silenced and not used anymore and the functions have moved to new locations, in particular this naive implanted substrate. And this way progressively regenerate the neocortex in that area.
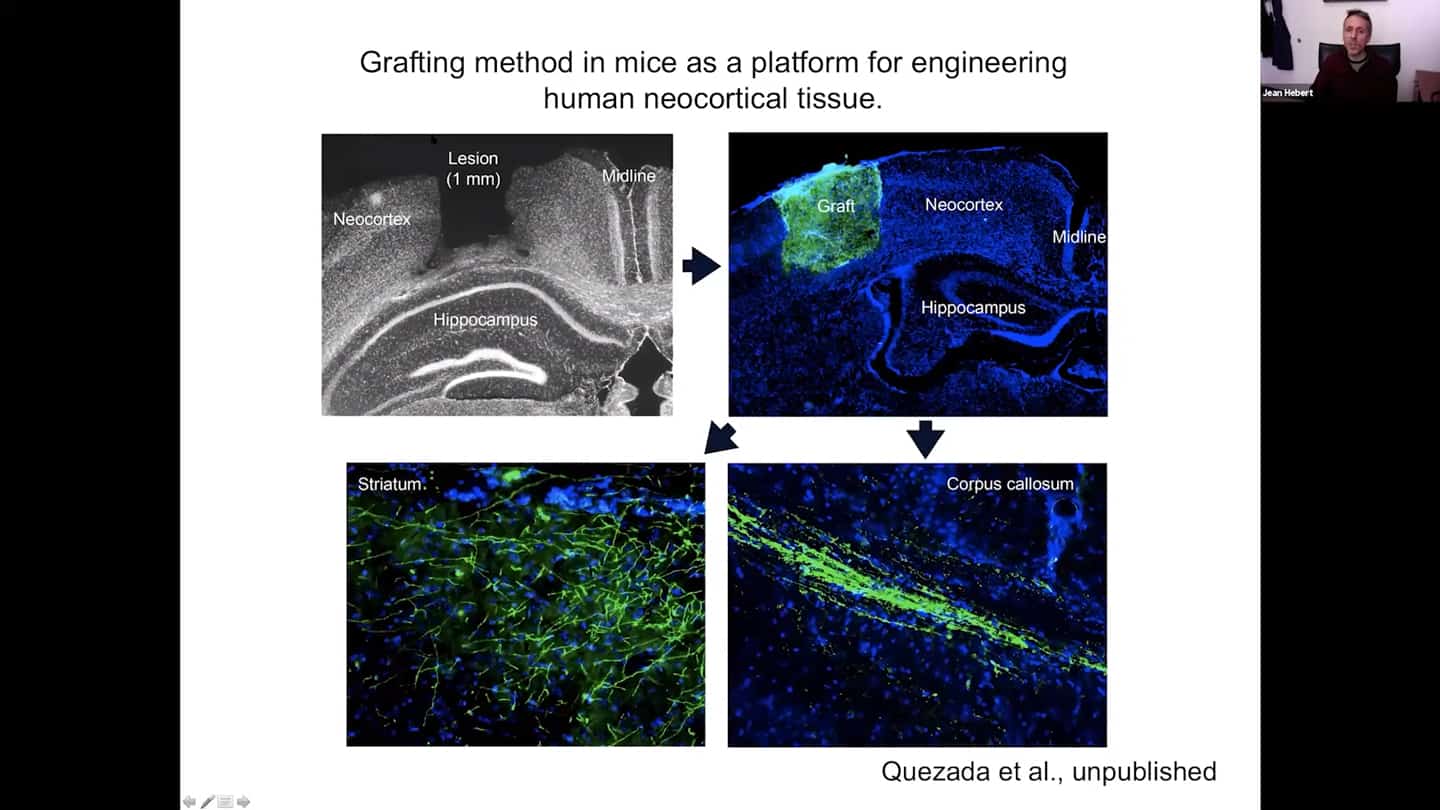
- We are trying that in mice – basically doing a lesion and using it as a platform for rebuilding functional human neocortical tissue. You can see the lesions and graft on the images.
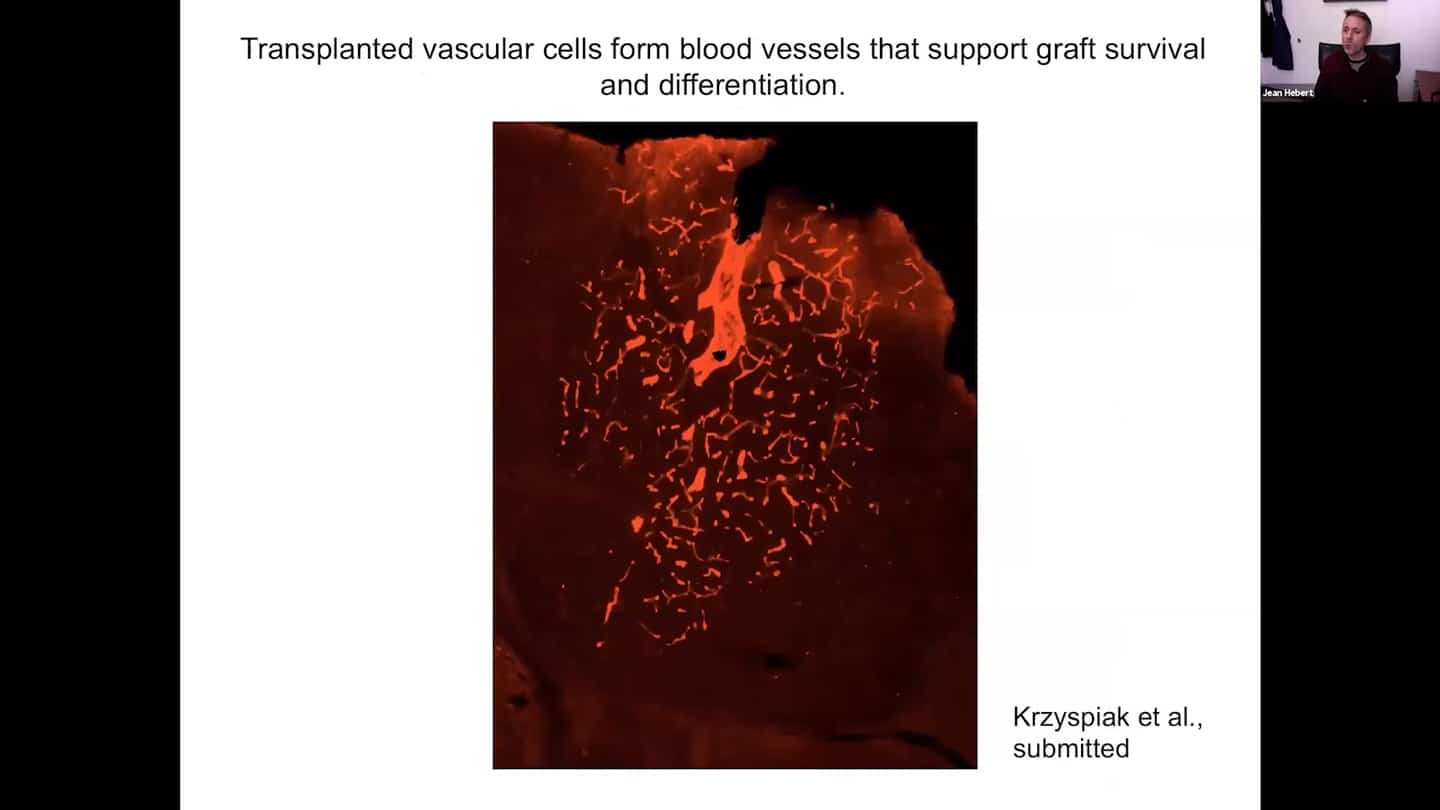
- When vascular cells are included in the grafts which is important to maintain the health and function of these grafts, we find that the vascular cells form blood vessels.
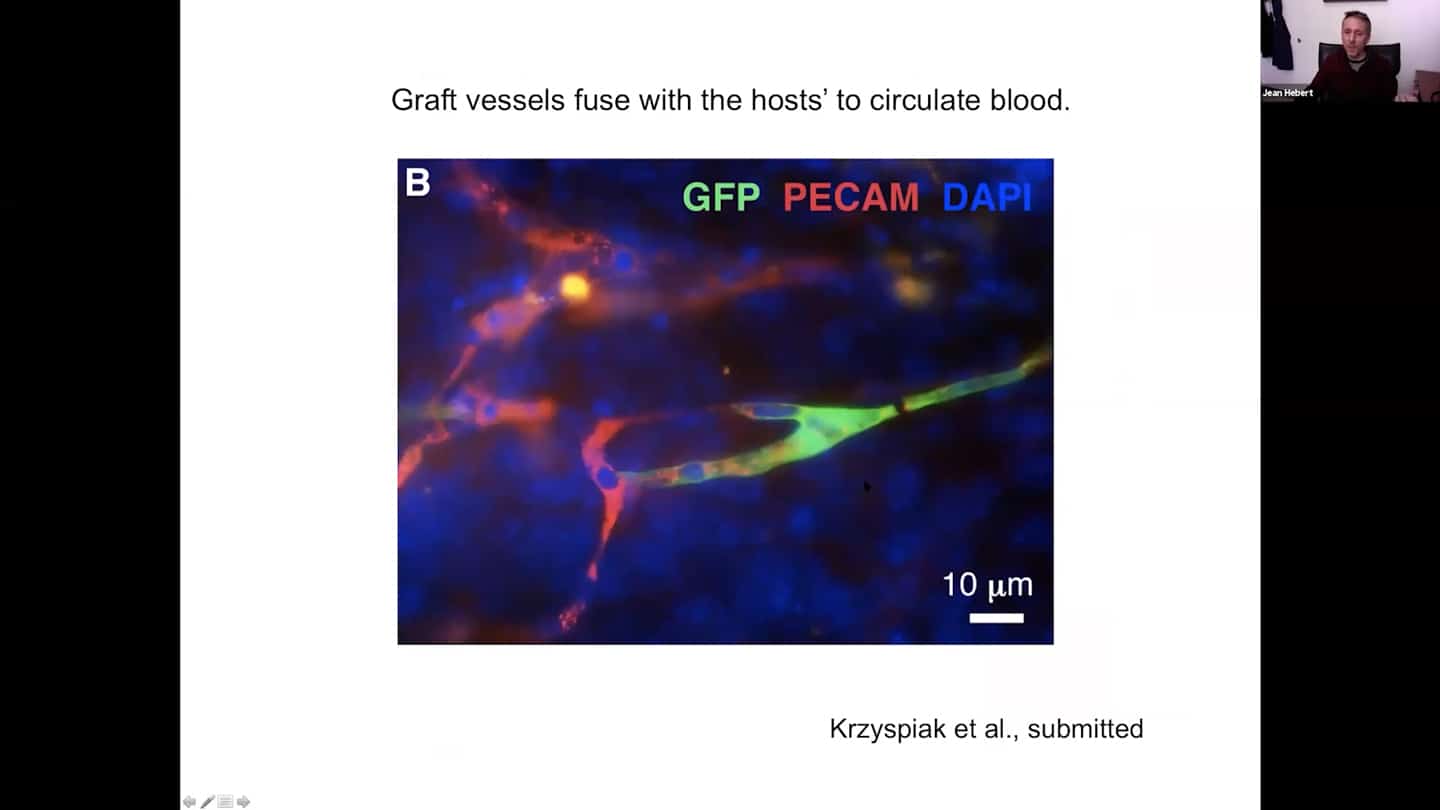
- Graft vascular cells host with hosts’ to circulate blood.
- And we can measure blood circulation within them in live animals that have grafts using two photon microscopy.
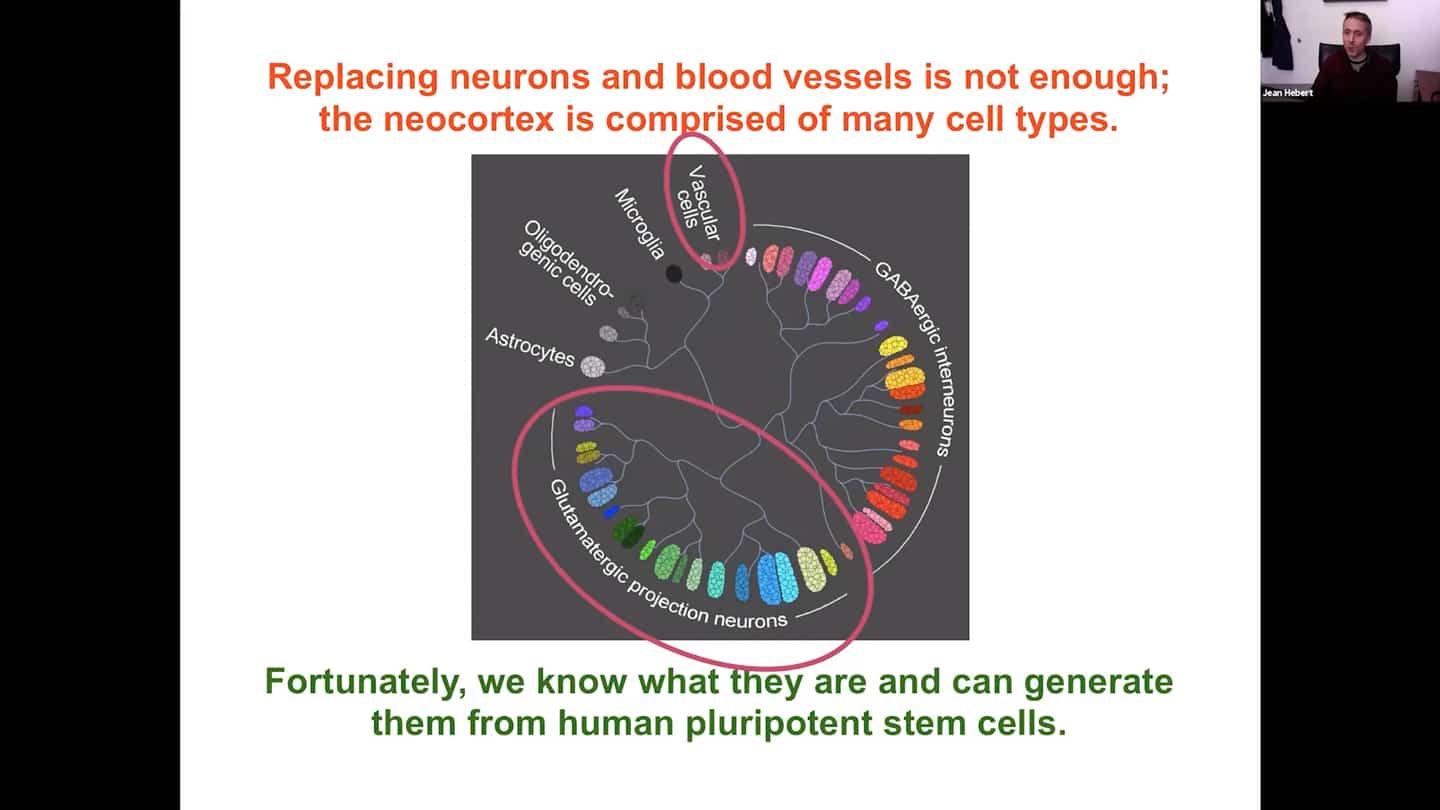
- All that is great, however there’s still a lot that needs to be done before this grafted tissue is functional. One thing that’s missing is a lot of cell types. Replacing neurons and blood vessels is not enough; the neocortex is comprised of many cell types. We are now working on including many other cell types, which we fortunately know how to generate from human embryonic stem cells. The protocols are not perfect, but good enough for testing.
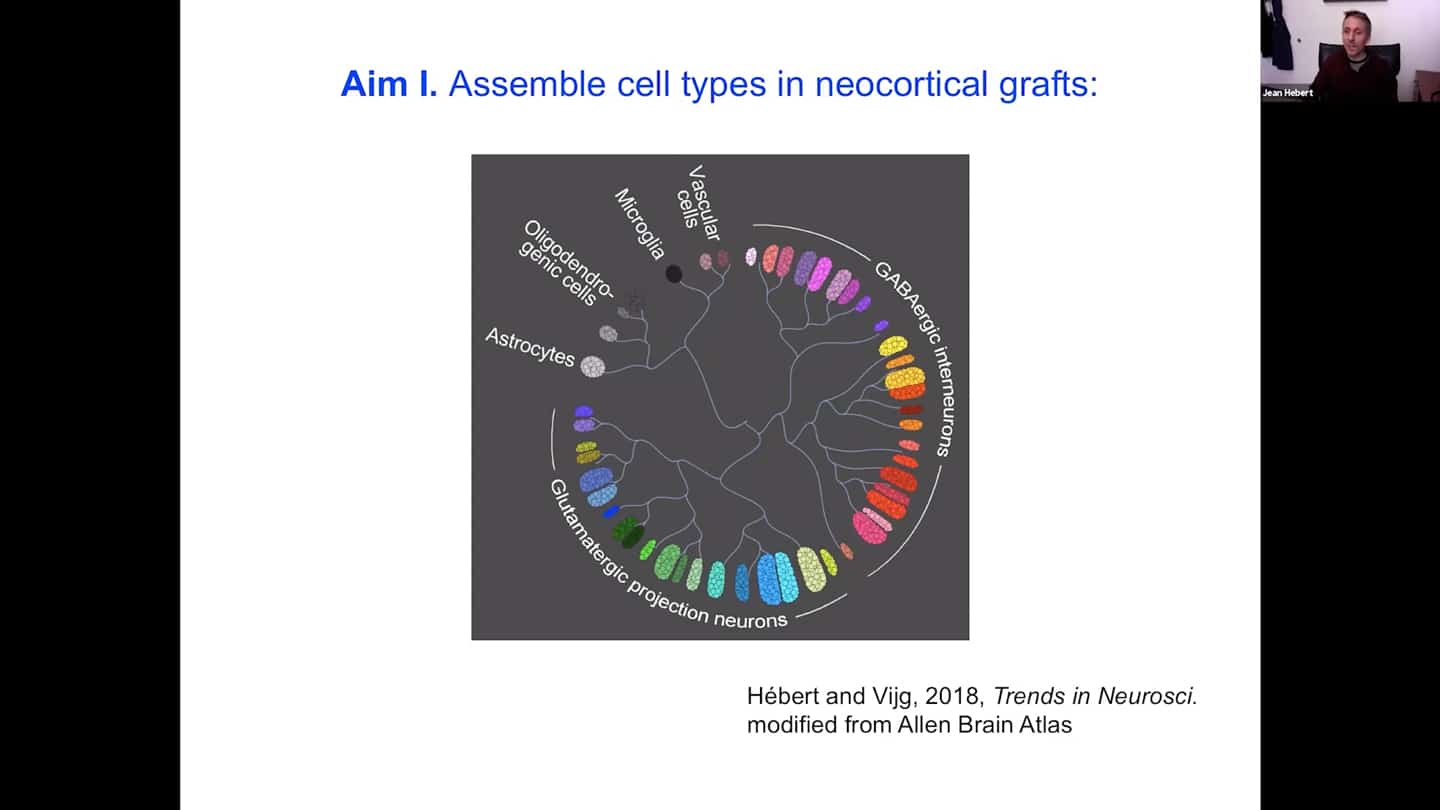
- The first aim is to assemble all the cell types we need to have a complete cortex. It’s not just to put them all in there together, they need to be organized.
- There’s a certain cytoarchitecture for a proper connectivity of the neurons in the cortex. Different layers have different densities with different identities or cell types. So we’re developing a scaffold that supports survival, differentiation, and integration of both neurons and vascular cells, whether human or mouse.
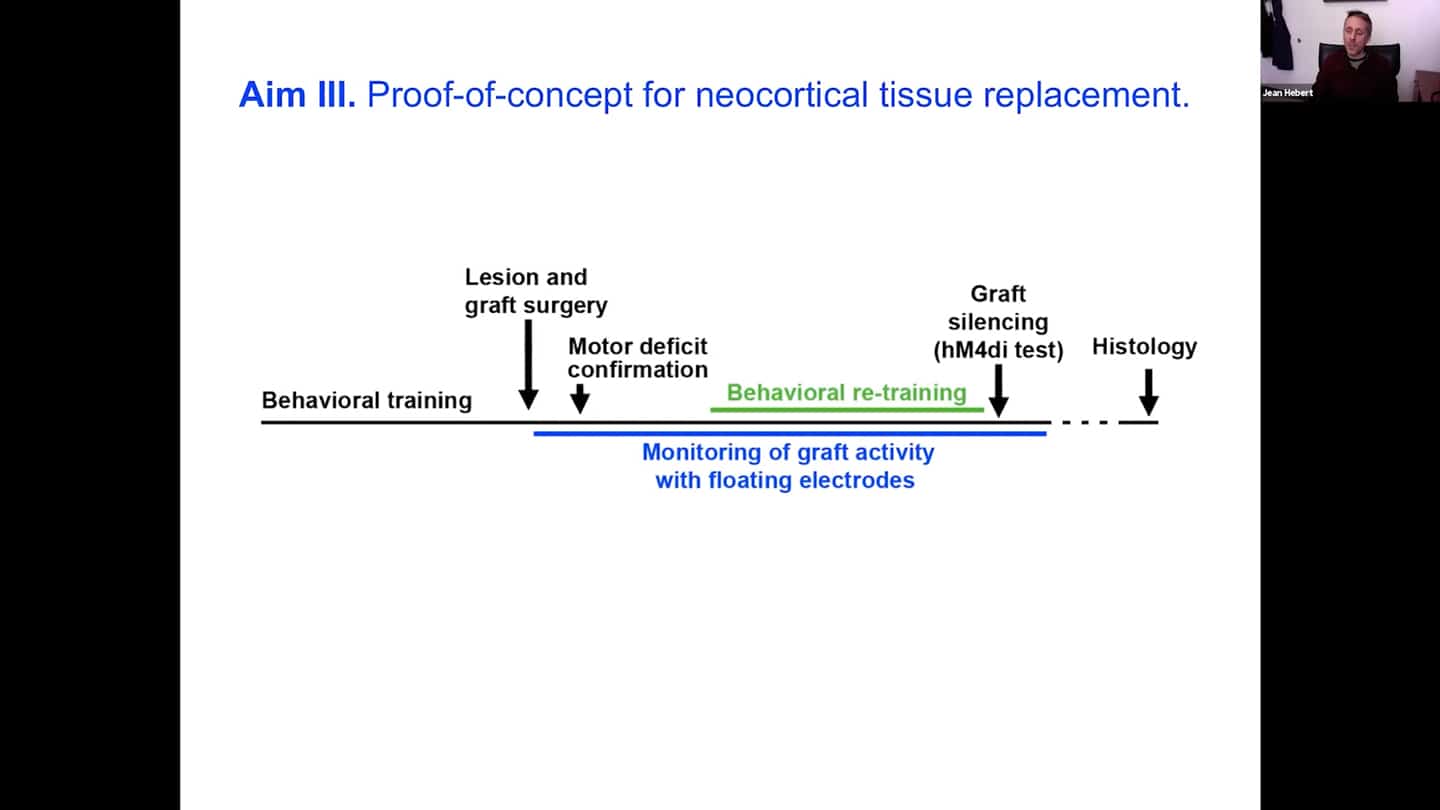
- Equally important thing that we want to do in parallel is to show a proof of principle for neocortical tissue replacement with an experiment. The way we’re doing that is that we train an animal to do a particular task that requires multiple parts of the neocortex.The task needs to go through one small part of the neocortex, the motor cortex. Then what we want to do is to lesion that part out and put their graft in and see whether the removal of the motor cortex created the expected functional deficit. And with the help of electrodes inside the graft, we can see whether over time the graft differentiates enough so with behavioral retraining the deficit is fixed by re-routing the function through that implanted and now activated graft. Once that happens, we can silence the graft using a transient channel with a drug, and show that the animals cannot do the task again, and then see it activate again and see them do the task again once the drug clears out. And we can do this several times, which will be the first demonstration that neocortical tissue replacement is possible. This has not been shown yet, so it’s an important landmark experiment, which is why it is very important to generate interest and investment.
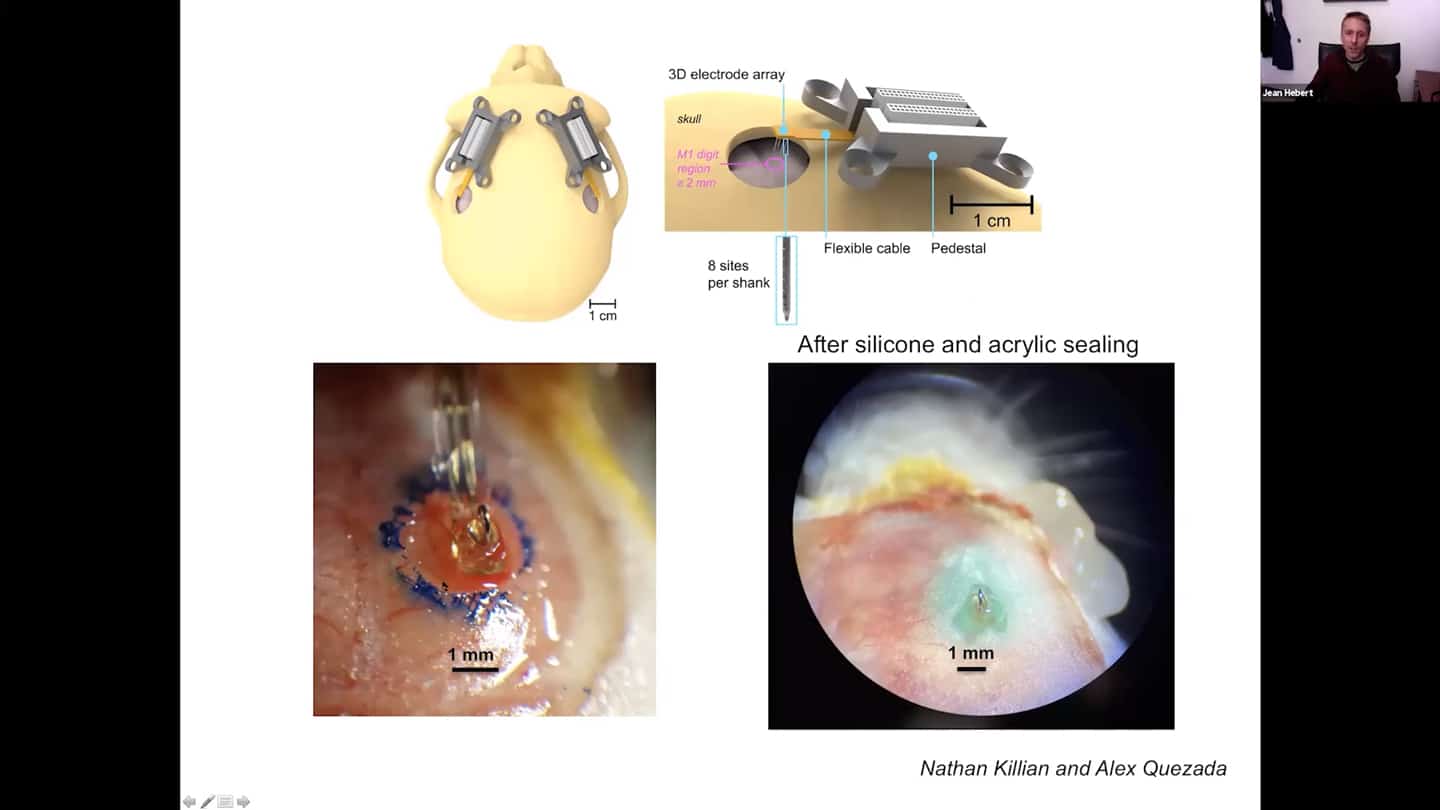
- This is how our floating electrodes look like, we are getting all the tools ready for the experiment.
- Here are the people who are collaborating on it. Unfortunately most of us do it part time, we need to make it a full time project to advance it properly.
Q&A
This is such an important area of research and replacement of brain cells is the way to go. Do you think it’s important/feasible to engineer the brain cells to be refractory to inflammatory signals (NFKB signalling)? We can’t really change the microenvironment, but maybe we can change and improve the cells to be refractory to the environment?
- Absolutely, these cells are grown in culture, you can modify them any way you want. So if we have the knowledge to make them better before grafting them, we certainly can, and I assume we certainly would.
Do the grafts put out the signals that ischemic tissues put out that attract vascularity?
- In our scaffold we put factors to encourage vascularization. Their first paper shows that with stroke as the first target model, which would also be the first clinical target (not aging), it does vascularize well in the ischemic environment. There might be something in the damaged part, maybe the inflammation, that encourages it.
Is there reason to believe that – if a patient has advanced Alzheimer’s for example – will newly grafted tissue serve to remediate such system conditions, or will the newly grafted tissue quickly become similarly corrupted due to the surrounding environment? Not expecting a definitive answer, of course, just curious if there is reason for optimism in this regard.
- We don’t know, but there is a reason for optimism. We already have a lot of data for Parkinson’s. The grafts of stem cells of fetal tissue, after two decades in post mortem analysis, the cells were still there and the grafted neurons didn’t show the deterioration that the hosts didn’t show. So it isn’t 100%, we know that, but it certainly provides some time. So the same thing might be true for Alzheimer’s.
- The other thing to keep in mind is the inherent nature of plasticity of neocortex. There is always competition for function in neocortex, so whenever we need a function, that’s the function that wins out. If you put a new tissue into the brain even if it is diseased, it will be used and rerouted, so it will definitely be effective at least for some time, and then you can put some improvements into the graft as discussed before, you could address that so it is not happening in this grafts.
The pessimistic case would be that with the buildup of the damage, it will be also necessary to address the generation of the damage itself, because the damage will spread really fast once there’s a lot of it (like senescent cells, misfolded proteins), as we already see it in recent studies, therefore just adding cells won’t be enough.
- We will definitely need to look at whether the intracellular or extracellular aggregates and damage travels to new tissues, not sure whether these experiments were actually done with Alzheimer’s. Either way, in the final regeneration strategy, we will definitely need to be able to address the whole brain and replace the vascular plexus and other parts of the brain where damage accumulates as well.
This is obviously a long term project. Could you define a clear goal that a funder could latch onto for sponsoring an institute project? An inspiring and motivating milestone with a specific goal to achieve in a number of years perhaps? And how much would that cost?
- We do have a development plan with milestones. Right now for 5 years it would cost between $10-$50 million, based on the commitment.
How much have you done studies on tumors and mapping how they are destroying the tissue? How much remapping is there? Is plasticity the main thing why you think this approach would make sense? There was a lot of effort with regenerating spinal cord and they got close, but are not there yet, perhaps because the plasticity in the spinal cord is worse.
- Plasticity is essential for this approach, yes. We’re working with neurosurgeons, who do quite a bit of brain tumor surgeries and mapping with electrodes of the function when removing tumors, also by functional MRI. And they even map where the functions have moved after the surgery, even with patients with recurrent tumors where the functions were already remapped before.
What kind of tissue are you going to replace with? Is it fetal tissue?
- With the tissue we’re going to engineer including the cytoarchitecture. We could start with human fetal neocortical tissue, we do have a few experiments testing that, but it’s not a very scalable nor applicable approach. We start with embryonic stem cells and generate the cells we need from them.
How does grafting new cells affect the biological age of the rest of the brain? The approach seems to have similarities to parabiosis or bone marrow stem cell transplants in that regard? There is always the question of fixing a particular problem and regenerating a subset of the tissue only for the subset of the tissue to be swiftly aged by the environment while speeding up aging of the rest of the tissue as well?
- In the longer term we will study that, we will be looking at things like epigenetics, senescent cells, protein aggregates, and much more.
- In more end-game sense, the way this procedure could be done foreseeably is to do functional replacements when the technology is ready. So do the whole auditory complex at once, instead of neocortex first and then hypothalamus, etc. So you develop together the young parts of the brain again, to mimic how it works normally.
Are you familiar with the work of Ellen Heber-Katz, who has been for quite a few years now inducing regeneration in mammalian organ systems without grafts but merely by manipulating the metabolic environment and activating basically latent amphibian regeneration capabilities in mammals? I believe they made progress not only with the heart and bone and skin, but even with the CNS and spinal cord without grafts. Maybe you could do it with the brain as well? Any interest in that?
- That area also seems to have promise, it’s very similar in a way – you’re removing damaged tissue and getting new tissue there. But it’s not known yet whether the regenerated tissue in those mice is epigenetically young. It also might be the case that the damage made to the organs is a necessary part of the regeneration. The body doesn’t recognize some tissue as “old” and just kill it and regenerate it. You have to damage the heart or punch the hole in the ear first probably. But would be happy to follow up on this thread.
Regarding to the possible disruptive complicated and perhaps damaging nature of the surgery, I wonder whether there might be a way to to introduce individual cells and use some sort of chemotaxis to get them to go where they should go without actually having to silence large parts of the brain and replace that with some sort of large amount of formed tissue? Or is that just something that’s gonna be too hard to get enough cells to go where they need to go and to differentiate and take on the role they need to take on structurally?
- Funny that you should ask, we have two projects in the lab, one is the first I presented today and the second one is exactly that – using cells that can disperse throughout the brain and getting them to convert to another cell type like the principal neurons of the neocortex. That could be used for many purposes. But it won’t get rid of the damage that’s there, so that’s why I favor this replacement approach for rejuvenation.
If an old/dysfunctional part of the brain is silenced — even slowly — and memory is at least partly distributed, and is located in that silenced part of the brain, isn’t there a risk that memories would be partly lost, depending on the part of the brain involved? (Of course it would depend on the nature of the distribution of memory, and whether the memories would “move” enough during the silencing.)
- We have a good idea of where the functions lie in the neocortex and how quickly they move. But you definitely don’t wanna silence too much at once, it’s gonna be a balance and a process.
What can people do for your group?
- All the collaborators are ready to go with this project, we have all the key experts in place who are willing to recruit more, we just need the funds to go at it full-time and get this thing going at a faster rate. The format of funding is pretty open, be it academia, or institute (which would be ideal), or some kind of hybrid of those, or anything else, those are all possible. The roadmap we need the funds for is here.
Seminar summary by Bolek Kerous.