Scanning probe manipulation of individual atoms and small molecules were amongst the early laboratory successes that helped bring broad scale attention to the feasibility and potential of nanoscale technologies, especially molecular fabrication.
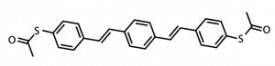
Basic manipulations of atoms and bonds by scanning probe have become familiar capabilities that follow similar protocols: the STM tip is precisely positioned relative to the molecule and then energy (tunneling current) is modulated to achieve particular operations, including scanning, translocation across the substrate surface, and making/breaking chemical bonds. Going a tremendous step beyond the basics, Wilson Ho* of University of California, Irvine and colleagues recently reported the selective formation of two distinct types of chemical bonds between gold adatoms and the sulfurs of 1,4-bis(4’-(acetylthio)styryl)benzene molecules (converting Ac-S-DSB-S-Ac to Au-S-DSB-S-Au) on a NiAl(110) surface.
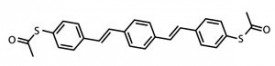
First, selective dissociation was achieved by successively positioning the STM tip at precise positions above each acetyl group and increasing sample bias to cause bond rupture, leaving a substrate-stabilized S-DSB-S molecule.
Each sulfur atom retained a lone pair and an unpaired electron which were available for bonding. Individual gold atoms were then manipulated toward the sulfurs from specific directions that targeted a particular electron group, and a pulse of energy was supplied from the STM tip to trigger discreet bonding. Bonding via the unpaired electron produced a covalent bond, while bonding via the lone pair produced a coordinate bond.
In this stepwise manner, the team was able to selectively produce molecules containing a covalent bond between one S-Au pair and a coordinate bond between the other S-Au pair, as well as molecules containing two covalently bound S-Au pairs or two coordinate pairs, for a total of nine distinct DSB-2S-2Au complexes to date.
Notably, the spatial location of the electron groups around the sulfur atoms appears to follow the symmetry of the DSB framework. Dr. Wilson Ho explained that, “Once the symmetry of the DSB is imaged and determined, we can infer the electron groups on the exposed sulfurs. The Au atoms can then be manipulated to form different types of bonds depending on whether they attach to the lone pair or the unpaired electron of the S atoms.”
This remarkable use of directionality for selective bond formation serves to further illustrate the fundamental accessibility of machine-guided synthesis: orbital overlap and energy barriers are key, and manipulation approaches will be increasingly understood and exploited as molecular fabrication technologies continue to develop.
*Sincere thanks to Dr. Wilson Ho for generous communications regarding this work
-Posted by Stephanie C