An advance in protein engineering targeted to better drug delivery methods or artificial vaccines is also an important step toward a general capability to build nanostructures by assembling designed protein domains in a designed rigid configuration. A hat tip to ScienceDaily for reprinting this UCLA news release written by Kim DeRose “Building molecular ‘cages’ to fight disease“:
UCLA biochemists have designed specialized proteins that assemble themselves to form tiny molecular cages hundreds of times smaller than a single cell. The creation of these miniature structures may be the first step toward developing new methods of drug delivery or even designing artificial vaccines.
“This is the first decisive demonstration of an approach that can be used to combine protein molecules together to create a whole array of nanoscale materials,” said Todd Yeates, a UCLA professor of chemistry and biochemistry and a member of the UCLA–DOE Institute of Genomics and Proteomics and the California NanoSystems Institute at UCLA.
Published June 1 in the journal Science [abstract], the research could be utilized to create cages from any number of different proteins, with potential applications across the fields of medicine and molecular biology.
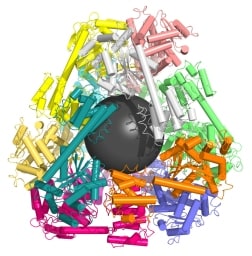
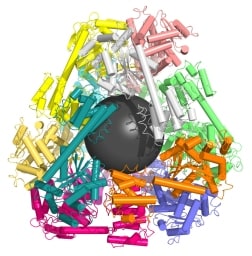
UCLA graduate student Yen-Ting Lai, lead author of the study, used computer models to identify two proteins that could be combined to form perfectly shaped three-dimensional puzzle pieces. Twelve of these specialized pieces fit together to create a molecular cage a mere fraction of the size of a virus.
“If you just connect two random proteins together, you expect to get an irregular network,” said Yeates, senior author of the study. “In order to control the geometry, the idea was to make a rigid link holding the two proteins in place as if they were parts of a toy puzzle.”
The specifically designed proteins intermesh to form a hollow lattice that could act as a vessel for drug delivery, he said.
“In principle, it would be possible to attach a recognition sequence for cancer cells on the outside of the cage, with a toxin or some other ‘magic bullet’ contained inside,” said Yeates. “That way, the drug could be delivered directly to certain targets like tumor cells.” …
A second breakthrough
A second paper co-authored by Yeates creates similarly designed molecular cages using multiple copies of the same protein as building blocks. The scientists control the shape of the cage by computing the sequence of amino acids necessary to link the proteins together at the correct angles. The research, also published today in Science [abstract], resulted from a collaboration between the UCLA team and professor David Baker [co-winner of the 2004 Foresight Institute Feynman Prize for Theoretical Molecular Nanotechnology] at the University of Washington.
This alternative method represents a more versatile approach because it requires only one type of protein to form a structure, Yeates said. However, devising different kinds of links between the identical proteins remains a major challenge. Lead author Neil King, a postdoctoral scholar at the University of Washington and a former student of Yeates, took the numerous computer-generated possibilities and tested each version experimentally until he found one which produced the right behavior.
The first paper reported a tetrahedral supramolecular 12-subunit cage about 16 nm in diameter, with an open center 5 nm in diameter. Each subunit comprised a trimer of one protein and a dimer of a different protein, fused together in a specified geometry. The second paper used trimers of a single protein as building blocks:
… to design a 24-subunit, 13-nm diameter complex with octahedral symmetry and a 12-subunit, 11-nm diameter complex with tetrahedral symmetry. The designed proteins assembled to the desired oligomeric states in solution, and the crystal structures of the complexes revealed that the resulting materials closely match the design models. The method can be used to design a wide variety of self-assembling protein nanomaterials.
Taken together, these two papers document a major advance in designing proteins to use as atomically precise building blocks.
—James Lewis, PhD