Among the specific recommendations of the 2007 Productive Nanosystems Technology Roadmap is the development of modular molecular composite nanosystems (MMCNs) in which “million-atom-scale DNA frameworks with dense arrays of distinct, addressable, [atomically precise] binding sites” provide scaffolds for organizing various nanoscale functional components (page x of Executive Summary of Productive Nanosystems: A Technology Roadmap PDF). Despite the obvious advantages of large DNA scaffolds, other possibilities are conceivable. Would protein scaffolds engineered for tissue engineering or other applications be suitable? A hat tip to KurzweilAI.net for reporting this news release from UC Davis “Engineering self-assembling amyloid fibers“:
Nature has many examples of self-assembly, and bioengineers are interested in copying or manipulating these systems to create useful new materials or devices. Amyloid proteins, for example, can self-assemble into the tangled plaques associated with Alzheimer’s disease — but similar proteins can also form very useful materials, such as spider silk, or biofilms around living cells. Researchers at UC Davis and Rice University have now come up with methods to manipulate natural proteins so that they self-assemble into amyloid fibrils. The paper is published online by the journal ACS Nano.
“These are big proteins with lots of flat surfaces suitable for functionalization, for example to grow photovoltaics or to attach to other surfaces,” said Dan Cox, a physics professor at UC Davis and coauthor on the paper. They could be used as “scaffolding” for tissue engineering, and potentially could be programmed so that other particles or proteins could be attached in specific locations or arrays. Amyloids are also tough: they can withstand boiling, attack by digestive proteins and ultraviolet radiation.
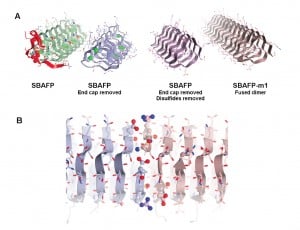
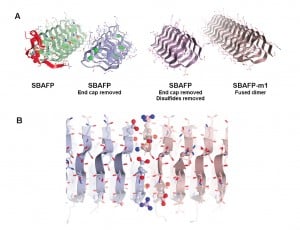
Maria Peralta, a graduate student in chemistry at UC Davis, and colleagues made the amyloid fibrils by tweaking natural “antifreeze” proteins from ryegrass and an insect, spruce budworm. These proteins allow some plants and animals to withstand very cold temperatures by preventing the growth of ice crystals, but they do not naturally self-assemble into larger structures.
The researchers removed cap structures from the end of the antifreeze proteins. They were then able to let them self-assemble into fibrils with predictable heights, a potential new material for bioengineering.
For a roadmap effort to guide progress toward a common goal, it would be useful to review progress in relevant technologies periodically to assess if changes are needed to take advantage of what has been learned in the interim. For good or for ill, progress toward atomically precise manufacturing currently may depend upon progress in various technologies that are primarily pursued for other purposes, such as tissue engineering or photovoltaics, and pursued by independent teams of researchers advancing independent goals. To the extent to which these various goals are all dependent on increased ability to program the structure of matter to atomic precision, simultaneous progress toward all of these goals may be possible.
—James Lewis, PhD